Abstract
Arising from M. M.Yartsev, M. P. Witter & N. Ulanovsky Nature 479, 103–107 (2011)10.1038/nature10583
Grid cells recorded in the medial entorhinal cortex (MEC) of freely moving rodents show a markedly regular spatial firing pattern whose underlying mechanism has been the subject of intense interest. Yartsev et al.1 report that the firing of grid cells in crawling bats does not show theta rhythmicity “causally disproving a major class of computational models” of grid cell firing that rely on oscillatory interference2,3,4,5,6,7. However, their data may be consistent with these models, with the apparent lack of theta rhythmicity reflecting slow movement speeds and low firing rates. Thus, the conclusion of Yartsev et al. is not supported by their data.
Similar content being viewed by others
Main
In oscillatory interference models, path integration is performed by velocity-dependent variation in the frequencies of theta-band oscillations, which combine to generate the grid-cell pattern2,3,4,6,7. In addition, learned associations to environmental sensory inputs (possibly mediated by place cells) ensure that grids are spatially stable over time and are sufficient to maintain firing in familiar environments2,3,8. In rats, the majority of grid cells show theta-modulated firing9,10, and the model predicts specific relationships between modulation frequency, running velocity and grid scale4, which have been verified in grid cells11 and in putative velocity-controlled oscillatory inputs identified as interneurons within the septohippocampal circuit7.
Yartsev et al.1 recorded the firing of grid cells from bats trained to crawl within the recording environment, a behaviour that they perform very slowly (a mean speed of 3.7 cm s−1 versus 17.6 cm s−1 in our rat data), often stopping entirely (supplementary figure 11 in ref. 1). The authors found grid cells with very low firing rates (a mean peak rate of 0.56 Hz versus 5.14 Hz in our data) and little significant theta modulation. However, matching movement speed is important for comparisons involving theta. At low speeds movement-related theta rhythmicity is strongly attenuated12 and the need for path integration is reduced. Equally importantly, low firing rates impede detection of theta rhythmicity (5–10 Hz), which requires periods containing plenty of spikes fired within tens to hundreds of milliseconds of each other (something that is absent in bat interspike interval histograms; supplementary figure 2b in ref. 1).
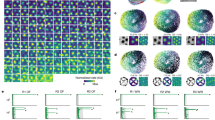
We examined whether differences in movement speeds and firing rates between the rat data and the bat data could explain the apparent lack of theta rhythmicity in bat grid cells. We took random samples of 25 cells from a representative data set of 85 grid cells recorded in rat MEC (Fig. 1a, bottom row), extracted periods of slow running to match bat movement speeds, and duplicated this data until it exceeded the duration of the longest bat trial (60 min). We then randomly discarded spikes to match the mean firing rates of each of the 25 published bat grid cells. From the 25 down-sampled rat cells matching each bat grid cell, we selected the one with the median theta index as representative. This process was repeated 10 times. Subjecting the 10 sets of 25 down-sampled cells to the analyses of Yartsev et al. produced a relative absence of theta rhythmicity (Fig. 1b, fourth row). So, if rats moved as slowly as bats and their grid cells fired as infrequently, rat grid cells would show bat levels of gridness (below the higher levels seen in rats), and theta modulation would be very hard to detect.
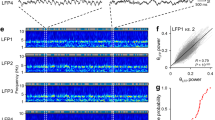
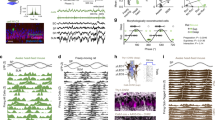
a, b, The firing of grid cells in rats (a) resembles grid-cell firing in bats1 if the rat data are down-sampled to match the low firing rates and slow movements of the bat data (b). c, d, The oscillatory interference model simulates theta-modulated grid cell firing in rats (c), and also apparently un-modulated grid-cell firing in bats when firing rates are reduced (d). a–d, Top row, example firing-rate maps (peak rate and gridness, above). Second row, example spike-train autocorrelograms. Third row, distributions of gridness scores. Fourth row, distributions of theta modulation (theta index). Grid cells have gridness > 0.33 (red line). ‘Theta-modulated cells’ have a theta index of ≥ 5 (red line). The theta index exceeded the 95th percentile for that cell’s temporally shuffled spike times for 58% of rat cells (a) but only for 2% of cells down-sampled to match the bat data (b; averaged over 10 samples of 25 cells). This rises to 14% if speed is not down-sampled, 20% if only the 25 most strongly theta-modulated rat cells are used and 72% for the 25 most strongly theta-modulated cells, if speed is unmatched. However, we do not consider this last cell population to be comparable to the bat grid cells because of the pre-selection of only the most strongly theta-modulated cells and the difference in movement speed between running rats and crawling bats. Theta index, gridness and shuffling follow ref. 1 (in which theta index is theta power divided by mean power 0–50 Hz), except for a, bottom row, which shows theta index calculated following ref. 13 (that is, theta power divided by mean power 0–125 Hz), giving higher values that match the proportions of theta-modulated cells in ref. 13 (which range from 62% in layer V, where most bat cells were recorded, to 90% in layer III). e, Schematic showing how theta-modulated inhibitory spike trains (top, black ticks) drive the grid cell’s membrane potential (middle, black trace), producing spikes when exceeding a threshold (middle, red dashed line). Spatial firing fields (bottom) are defined by constructive interference (top, grey lines show theta modulation; middle, grey line shows the resulting interference pattern), but the underlying oscillations are undetectable at low firing rates (see Methods for details).
Most importantly, to disprove the model requires knowing how much theta rhythmicity it predicts in low-firing-rate cells. Simulations (using code adapted from ref. 7) with strong theta modulation and typical firing rates for rats (Fig. 1c) also lack significant theta modulation when firing rates are reduced to bat levels (Fig. 1d, fourth row). Although spatially modulated firing is driven by interference between theta-modulated inputs, the theta rhythmicity is undetectable in low-rate spike trains (Fig. 1e).
Local field potentials and multi-unit activity were also reported in bats1, but these reflect the physical arrangement and coherence of populations of cells, which may vary between species and are not addressed by the model (although spatially offset grids require phase-offset oscillators7, suggesting no overall phase preference in the model). Finally, consistent with the model, grids might be set up through oscillatory interference during the initial training of the bats to not fly out of the box (by physically blocking from above), and maintained (at lower firing rates) by learned sensory associations during subsequent slow crawling in the now highly familiar box.
Methods
The activity of 85 grid cells was recorded from superficial and deep layers of rat MEC during 20 min foraging in 1-m2 arenas using standard procedures8. Random samples of 25 cells were speed matched by removing periods of fast running, retaining periods of ≥0.5 s, until the median speed was 3.7 cm s−1. Speed-matched data were duplicated and concatenated to exceed the duration of the longest bat trial (60 min). Cell firing rates were down-sampled by randomly removing spikes, in turn, to match the mean firing rate of each of the 25 bat grid cells (mean rate taken as 25% of the peak rates found by Yartsev et al. (range of mean rates, 0.03–0.40 Hz)). Spike-train autocorrelograms combined the individual autocorrelograms from each period of slow running11 and were mean-normalized to avoid low-frequency power reducing the theta index (compare with figure 4g in ref. 1). Grid cells were simulated as leaky integrate-and-fire neurons (time constant 20 ms) receiving three oscillatory inhibitory spike trains7 (Poisson processes with rate 50 + 30cos(2πft), in which frequency (f) varies around 8 Hz according to running velocity, with a peak inhibitory synaptic conductance14 of 14 pS) and a noisy persistent excitatory current sampled from N(m,2m), in which m = 336 nA for low firing rates and m = 436 nA for high rates (mean peak rates are 0.48 Hz and 5.11 Hz, respectively).
References
Yartsev, M. M., Witter, M. P. & Ulanovsky, N. Grid cells without theta oscillations in the entorhinal cortex of bats. Nature 479, 103–107 (2011)
Burgess, N., Barry, C., Jeffery, K. J. & O'Keefe, J. A grid and place cell model of path integration utilizing phase precession versus theta. http://cdn.f1000.com/posters/docs/225 (Computational Cognitive Neuroscience Conference Poster, 2005)
Burgess, N., Barry, C. & O’Keefe, J. An oscillatory interference model of grid cell firing. Hippocampus 17, 801–812 (2007)
Burgess, N. Grid cells and theta as oscillatory interference: theory and predictions. Hippocampus 18, 1157–1174 (2008)
Giocomo, L. M., Zilli, E. A., Fransen, E. & Hasselmo, M. E. Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing. Science 315, 1719–1722 (2007)
Hasselmo, M. E. Grid cell mechanisms and function: contributions of entorhinal persistent spiking and phase resetting. Hippocampus 18, 1213–1229 (2008)
Welday, A. C., Shlifer, I. G., Bloom, M. L., Zhang, K. & Blair, H. T. Cosine directional tuning of theta cell burst frequencies: evidence for spatial coding by oscillatory interference. J. Neurosci. 31, 16157–16176 (2011)
Barry, C., Hayman, R., Burgess, N. & Jeffery, K. J. Experience-dependent rescaling of entorhinal grids. Nature Neurosci. 10, 682–684 (2007)
Hafting, T., Fyhn, M., Molden, S., Moser, M. B. & Moser, E. I. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806 (2005)
Hafting, T., Fyhn, M., Bonnevie, T., Moser, M. B. & Moser, E. I. Hippocampus-independent phase precession in entorhinal grid cells. Nature 453, 1248–1252 (2008)
Jeewajee, A., Barry, C., O’Keefe, J. & Burgess, N. Grid cells and theta as oscillatory interference: electrophysiological data from freely moving rats. Hippocampus 18, 1175–1185 (2008)
O'Keefe, J. The Hippocampus Book (eds Andersen, P., Morris, R. G. M., Amaral, D. G., Bliss, T. V. P. & O’Keefe, J. ) 475–548 (Oxford Univ. Press, 2006)
Boccara, C. N. et al. Grid cells in pre- and parasubiculum. Nature Neurosci. 13, 987–994 (2010)
Dayan, P. & Abbott, L. F. Theoretical Neuroscience 180–183 (MIT Press, 2001)
Author information
Authors and Affiliations
Contributions
C.B. analysed and collected experimental data; D.B. performed simulations; N.B. supervised analyses and simulations; and N.B., C.B., J.O. and D.B. contributed to writing.
Corresponding author
Ethics declarations
Competing interests
Declared none.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Barry, C., Bush, D., O’Keefe, J. et al. Models of grid cells and theta oscillations. Nature 488, E1 (2012). https://doi.org/10.1038/nature11276
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11276
This article is cited by
-
Scalp recorded theta activity is modulated by reward, direction, and speed during virtual navigation in freely moving humans
Scientific Reports (2022)
-
Grid cell remapping under three-dimensional object and social landmarks detected by implantable microelectrode arrays for the medial entorhinal cortex
Microsystems & Nanoengineering (2022)
-
Dynamic regulation of interregional cortical communication by slow brain oscillations during working memory
Nature Communications (2019)
-
Membrane potential dynamics of grid cells
Nature (2013)
-
From A to Z: a potential role for grid cells in spatial navigation
Neural Systems & Circuits (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.