Abstract
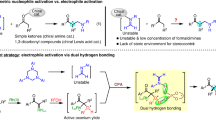
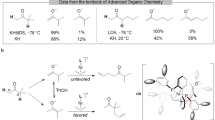
The enantioselective allylation of ketones is a problem of fundamental importance in asymmetric reaction design, especially given that only a very small number of methods can generate tertiary carbinols. Despite the vast amount of attention that synthetic chemists have given to this problem1,2,3,4,5,6,7,8, success has generally been limited to just a few simple ketone types. A method for the selective allylation of functionally complex ketones would greatly increase the utility of ketone allylation methods in the chemical synthesis of important targets. Here we describe the operationally simple, direct, regioselective and enantioselective allylation of β-diketones. The strong tendency of β-diketones to act as nucleophilic species was overcome by using their enol form to provide the necessary Brønsted-acid activation. This reaction significantly expands the pool of enantiomerically enriched and functionally complex tertiary carbinols that may be easily accessed. It also overturns more than a century of received wisdom regarding the reactivity of β-diketones.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Data deposits
X-ray crystallographic data have been deposited in the Cambridge Crystallographic Data Centre database (http://www.ccdc.cam.ac.uk/) under accession code CCDC 874744.
References
Nakamura, M., Hirai, A., Sogi, M. & Nakamura, E. Enantioselective addition of allylzinc reagent to alkynyl ketones. J. Am. Chem. Soc. 120, 5846–5847 (1998)
Waltz, K. M., Gavenonis, J. & Walsh, P. J. A simple, reliable, catalytic asymmetric allylation of ketones. Angew. Chem. Int. Edn 41, 3697–3699 (2002)
Wu, T. R., Shen, L. & Chong, J. M. Asymmetric allylboration of aldehydes and ketones using 3,3′-disubstitutedbinaphthol-modified boronates. Org. Lett. 6, 2701–2704 (2004)
Canales, E., Prasad, K. G. & Soderquist, J. A. B-Allyl-10-Ph-9-borabicyclo[3.3.2]decanes: strategically designed for the asymmetric allylboration of ketones. J. Am. Chem. Soc. 127, 11572–11573 (2005)
Wadamoto, M. & Yamamoto, H. Silver-catalyzed asymmetric Sakurai–Hosomi allylation of ketones. J. Am. Chem. Soc. 127, 14556–14557 (2005)
Miller, J. J. & Sigman, M. S. Design and synthesis of modular oxazoline ligands for the enantioselective chromium-catalyzed addition of allyl bromide to ketones. J. Am. Chem. Soc. 129, 2752–2753 (2007)
Barnett, D. S., Moquist, P. N. & Schaus, S. E. The mechanism and an improved asymmetric allylboration of ketones catalyzed by chiral biphenols. Angew. Chem. Int. Edn 48, 8679–8682 (2009)
Shi, S.-L., Xu, L.-W., Oisaki, K., Kanai, M. & Shibasaki, M. Identification of modular chiral bisphosphines effective for Cu(I)-catalyzed asymmetric allylation and propargylation of ketones. J. Am. Chem. Soc. 132, 6638–6639 (2010)
Robak, M. T., Herbage, M. A. & Ellman, J. A. Synthesis and applications of tert-butanesulfinimide. Chem. Rev. 110, 3600–3740 (2010)
Pettit, G. R. et al. Isolation and structure of spongistatin 1. J. Org. Chem. 58, 1302–1304 (1993)
Fusetani, N., Shinoda, K. & Matsunaga, S. Cinachyrolide A: a potent cytotoxic macrolide possessing two spiro ketals from marine sponge Cinachyra sp. J. Am. Chem. Soc. 115, 3977–3981 (1993)
Kobayashi, M. et al. Altohyrtin A, a potent anti-tumor macrolide from the Okinawan marine sponge Hyrtios altum . Tetrahedr. Lett. 34, 2795–2798 (1993)
Gaich, T. & Baran, P. S. Aiming for the ideal synthesis. J. Org. Chem. 75, 4657–4673 (2010)
Deng, D.-L. & Lu, Z.-H. Monoallylation of symmetric diketones in the presence of water by allylic bromide and metallic zinc. Chin. Chem. Lett. 5, 173–176 (1994)
Kira, M., Sato, K., Sekimoto, K., Gewald, R. & Sakurai, H. Stereoselective allylation of β-hydroxy- and β-amino-α,β-enones with allyltrifluorosilane/triethylamine systems. Chem. Lett. 1995, 281–282 (1995)
Marton, D., Stivanello, D. & Tagliavini, G. Allylstannation of α-, β- and γ-diketones mediated by allylbutyltin halides: Bu2(CH2 = CHCH2)SnCl and Bu(CH2 = CHCH2)SnCl2 . J. Organomet. Chem. 540, 77–81 (1997)
Leighton, J. L. Powerful and versatile silicon Lewis acids for asymmetric chemical synthesis. Aldrichim. Acta 43, 3–12 (2010)
Kim, H., Ho, S. & Leighton, J. L. A more comprehensive and highly practical solution to enantioselective aldehyde crotylation. J. Am. Chem. Soc. 133, 6517–6520 (2011)
Burns, N. Z., Hackman, B. M., Ng, P. Y., Powelson, I. A. & Leighton, J. L. The enantioselective allylation and crotylation of sterically hindered and functionalized aryl ketones: convenient access to unusual tertiary carbinol structures. Angew. Chem. Int. Edn 45, 3811–3813 (2006)
Hackman, B. M., Lombardi, P. J. & Leighton, J. L. Highly diastereo- and enantioselective reagents for aldehyde crotylation. Org. Lett. 6, 4375–4377 (2004)
Spletstoser, J. T., Zacuto, M. J. & Leighton, J. L. Tandem silylformylation–crotylsilylation/Tamao oxidation of internal alkynes: a remarkable example of generating complexity from simplicity. Org. Lett. 10, 5593–5596 (2008)
Pinnavaia, T. J., Collins, W. T. & Howe, J. J. Triorganosilicon acetylacetonates. Enol ether isomerism and stereochemical lability. J. Am. Chem. Soc. 92, 4544–4550 (1970)
Reich, H. J. & Murcia, D. A. Stereochemical studies of degenerate silyl rearrangements. Stereospecificity of the tropolone and acetylacetone trialkylsilyl ether rearrangements. J. Am. Chem. Soc. 95, 3418–3420 (1973)
Pinnavaia, T. J. & McClarin, J. A. Rearrangements of 1-acetylacetonato-1-methyl-1-silacyclobutane via internal nucleophilic displacement. J. Am. Chem. Soc. 96, 3012–3013 (1974)
McClarin, J. A., Schwartz, A. & Pinnavaia, T. J. 1,5-Migrations of silicon between oxygen centers in silyl β-diketones. J. Organomet. Chem. 188, 129–139 (1980)
Seeman, J. I. Effect of conformational change on reactivity in organic chemistry. Evaluations, applications, and extensions of Curtin–Hammett Winstein–Holness kinetics. Chem. Rev. 83, 83–134 (1983)
Mirarchi, D. & Ritchie, G. L. D. Solution-state conformations of 2-fluoro-, 2-chloro- and 2-bromo-acetophenone: a diploe moment and Kerr effect study. J. Mol. Struct. 118, 303–310 (1984)
Kulhánek, J., Böhm, S., Palát, K., Jr & Exner, O. Steric inhibition of resonance: revision of the principle on the electronic spectra of methyl-substituted acetophenones. J. Phys. Org. Chem. 17, 686–693 (2004)
Cheng, C. L. & Ritchie, G. L. D. Conformations of the formyl-, acetyl-, and benzoyl-pyridines. J. Chem. Soc. 2, 1461–1465 (1973)
Ramachandran, P. V., Gong, B., Brown, H. C. & Francisco, J. S. Relationship between the structure and enantioselectivity in the asymmetric reduction of 2’,6’-disubstituted acetophenones with DIP-chlorideTM. An ab initio study. Tetrahedr. Lett. 45, 2603–2605 (2004)
Acknowledgements
This work was supported by a grant from the National Institute of General Medical Sciences (GM58133). W.A.C. was supported by a Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship. We thank the US National Science Foundation (CRIF-0840451) for acquisition of a 400 MHz NMR spectrometer. We thank our colleagues G. Parkin and W. Sattler for an X-ray structure analysis (see the Supplementary Information), and the US National Science Foundation (CHE-0619638) for acquisition of an X-ray diffractometer.
Author information
Authors and Affiliations
Contributions
W.A.C. planned and did the vast majority of the experimental work. S.K.R. did the experiments that established the validity of the idea and optimized the allylation of acetylacetone. J.L.L. conceived and directed the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-80, Supplementary Methods, Supplementary Table 1, and Supplementary References. (PDF 14180 kb)
Rights and permissions
About this article
Cite this article
Chalifoux, W., Reznik, S. & Leighton, J. Direct and highly regioselective and enantioselective allylation of β-diketones. Nature 487, 86–89 (2012). https://doi.org/10.1038/nature11189
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11189
This article is cited by
-
Reactions at the end of a tether
Nature (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.