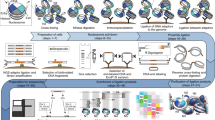
Abstract
The exact positions of nucleosomes along genomic DNA can influence many aspects of chromosome function. However, existing methods for mapping nucleosomes do not provide the necessary single-base-pair accuracy to determine these positions. Here we develop and apply a new approach for direct mapping of nucleosome centres on the basis of chemical modification of engineered histones. The resulting map locates nucleosome positions genome-wide in unprecedented detail and accuracy. It shows new aspects of the in vivo nucleosome organization that are linked to transcription factor binding, RNA polymerase pausing and the higher-order structure of the chromatin fibre.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Richmond, T. J. & Davey, C. A. The structure of DNA in the nucleosome core. Nature 423, 145–150 (2003)
Koslover, E. F., Fuller, C. J., Straight, A. F. & Spakowitz, A. J. Local geometry and elasticity in compact chromatin structure. Biophys. J. 99, 3941–3950 (2010)
Li, G. & Widom, J. Nucleosomes facilitate their own invasion. Nature Struct. Mol. Biol. 11, 763–769 (2004)
Raveh-Sadka, T., Levo, M. & Segal, E. Incorporating nucleosomes into thermodynamic models of transcription regulation. Genome Res. 19, 1480–1496 (2009)
Mao, C., Brown, C. R., Griesenbeck, J. & Boeger, H. Occlusion of regulatory sequences by promoter nucleosomes in vivo . PLoS ONE 6, e17521 (2011)
Li, B., Carey, M. & Workman, J. L. The role of chromatin during transcription. Cell 128, 707–719 (2007)
Petesch, S. J. & Lis, J. T. Rapid, transcription-independent loss of nucleosomes over a large chromatin domain at Hsp70 loci. Cell 134, 74–84 (2008)
Churchman, L. S. & Weissman, J. S. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature 469, 368–373 (2011)
Owen-Hughes, T. & Workman, J. L. Experimental analysis of chromatin function in transcription control. Crit. Rev. Eukaryot. Gene Expr. 4, 403–441 (1994)
Li, G., Levitus, M., Bustamante, C. & Widom, J. Rapid spontaneous accessibility of nucleosomal DNA. Nature Struct. Mol. Biol. 12, 46–53 (2005)
Lipford, J. R. & Bell, S. P. Nucleosomes positioned by ORC facilitate the initiation of DNA replication. Mol. Cell 7, 21–30 (2001)
Dorn, E. S. & Cook, J. G. Nucleosomes in the neighborhood: new roles for chromatin modifications in replication origin control. Epigenetics 6, 552–559 (2011)
Tachiwana, H. et al. Crystal structure of the human centromeric nucleosome containing CENP-A. Nature 476, 232–235 (2011)
Cole, H. A., Howard, B. H. & Clark, D. J. The centromeric nucleosome of budding yeast is perfectly positioned and covers the entire centromere. Proc. Natl Acad. Sci. USA 108, 12687–12692 (2011)
Dalal, Y., Furuyama, T., Vermaak, D. & Henikoff, S. Structure, dynamics, and evolution of centromeric nucleosomes. Proc. Natl Acad. Sci. USA 104, 15974–15981 (2007)
Beckmann, J. S. & Trifonov, E. N. Splice junctions follow a 205-base ladder. Proc. Natl Acad. Sci. USA 88, 2380–2383 (1991)
Schwartz, S., Meshorer, E. & Ast, G. Chromatin organization marks exon-intron structure. Nature Struct. Mol. Biol. 16, 990–995 (2009)
Tilgner, H. et al. Nucleosome positioning as a determinant of exon recognition. Nature Struct. Mol. Biol. 16, 996–1001 (2009)
Dingwall, C., Lomonossoff, G. P. & Laskey, R. A. High sequence specificity of micrococcal nuclease. Nucleic Acids Res. 9, 2659–2673 (1981)
Fan, X. et al. Nucleosome depletion at yeast terminators is not intrinsic and can occur by a transcriptional mechanism linked to 3′-end formation. Proc. Natl Acad. Sci. USA 107, 17945–17950 (2010)
Hörz, W. & Altenburger, W. Sequence specific cleavage of DNA by micrococcal nuclease. Nucleic Acids Res. 9, 2643–2658 (1981)
Chung, H. R. et al. The effect of micrococcal nuclease digestion on nucleosome positioning data. PLoS ONE 5, e15754 (2010)
Zhang, Y. et al. Intrinsic histone-DNA interactions are not the major determinant of nucleosome positions in vivo . Nature Struct. Mol. Biol. 16, 847–852 (2009)
Flaus, A., Luger, K., Tan, S. & Richmond, T. J. Mapping nucleosome position at single base-pair resolution by using site-directed hydroxyl radicals. Proc. Natl Acad. Sci. USA 93, 1370–1375 (1996)
Flaus, A. & Richmond, T. J. Base-pair resolution mapping of nucleosome positions using site-directed hydroxy radicals. Methods Enzymol. 304, 251–263 (1999)
Yager, T. D., McMurray, C. T. & van Holde, K. E. Salt-induced release of DNA from nucleosome core particle. Biochemistry 28, 2271–2281 (1989)
Widom, J. Role of DNA sequence in nucleosome stability and dynamics. Q. Rev. Biophys. 34, 269–324 (2001)
Thåström, A., Bingham, L. M. & Widom, J. Nucleosomal locations of dominant DNA sequence motifs for histone–DNA interactions and nucleosome positioning. J. Mol. Biol. 338, 695–709 (2004)
Teif, V. B. & Rippe, K. Predicting nucleosomes positions on the DNA: combining intrinsic sequence preferences and remodeler activities. Predicting nucleosome positions on the DNA. Nucleic Acids Res. 37, 5642–5655 (2009)
Field, Y. et al. Distinct modes of regulation by chromatin encoded through nucleosome positioning signals. PLoS Comput. Biol. 4, e1000216 (2008)
Floer, M. et al. A RSC/nucleosome complex determines chromatin architecture and facilitates activator binding. Cell 141, 407–418 (2010)
Cockell, M., Rhodes, D. & Klug, A. Location of the primary sites of micrococcal nuclease cleavage on the nucleosome core. J. Mol. Biol. 170, 423–446 (1983)
Albert, I. et al. Translational and rotational settings of H2A.Z nucleosomes across the Saccharomyces cerevisiae genome. Nature 446, 572–576 (2007)
Engeholm, M. et al. Nucleosomes can invade DNA territories occupied by their neighbors. Nature Struct. Mol. Biol. 16, 151–158 (2009)
Wang, J. P. & Widom, J. Improved alignment of nucleosome DNA sequences using a mixture model. Nucleic Acids Res. 33, 6743–6755 (2005)
Segal, E. et al. A genomic code for nucleosome positioning. Nature 442, 772–778 (2006)
Travers, A. A. & Klug, A. The bending of DNA in nucleosomes and its wider implications. Phil. Trans. R. Soc. Lond. B 317, 537–561 (1987)
Segal, E. & Widom, J. What controls nucleosome positions? Trends Genet. 25, 335–343 (2009)
Segal, E. & Widom, J. Poly(dA:dT) tracts: major determinants of nucleosome organization. Curr. Opin. Struct. Biol. 19, 65–71 (2009)
Thåström, A., Lowary, P. T. & Widom, J. Measurement of histone-DNA interaction free energy in nucleosomes. Methods 33, 33–44 (2004)
David, L. et al. A high-resolution map of transcription in the yeast genome. Proc. Natl Acad. Sci. USA 103, 5320–5325 (2006)
Schramm, L. & Hernandez, N. Recruitment of RNA polymerase III to its target promoters. Genes Dev. 16, 2593–2620 (2002)
Mavrich, T. N. et al. A barrier nucleosome model for statistical positioning of nucleosomes throughout the yeast genome. Genome Res. 18, 1073–1083 (2008)
Zhang, Z. et al. A packaging mechanism for nucleosome organization reconstituted across a eukaryotic genome. Science 332, 977–980 (2011)
Lohr, D. & van Holde, K. E. Organization of spacer DNA in chromatin. Proc. Natl Acad. Sci. USA 76, 6326–6330 (1979)
Routh, A., Sandin, S. & Rhodes, D. Nucleosome repeat length and linker histone stoichiometry determine chromatin fiber structure. Proc. Natl Acad. Sci. USA 105, 8872–8877 (2008)
Wang, J. P. et al. Preferentially quantized linker DNA lengths in Saccharomyces cerevisiae . PLoS Comput. Biol. 4, e1000175 (2008)
Dorigo, B. et al. Nucleosome arrays reveal the two-start organization of the chromatin fiber. Science 306, 1571–1573 (2004)
MacIsaac, K. D. et al. An improved map of conserved regulatory sites for Saccharomyces cerevisiae . BMC Bioinformatics 7, 113 (2006)
Acknowledgements
We dedicate this paper to J.W., who guided this project. We thank R. Holmgren, A. Matouschek, B. Meyer, R. Phillips, E. Segal and O. Uhlenbeck for comments and discussions. We are grateful to Northwestern University’s Genomic Core for all sequencing completed for this project. The work was supported by National Institutes of Health grants T32GM00806 (to K.B.), R01GM058617 (to J.W.), R01GM075313 (to J.-P.W.) and U54CA143869 (to J.W.).
Author information
Authors and Affiliations
Contributions
K.B. did all the experimental work. L.X. and J.-P.W. developed the algorithm and performed the analyses. K.B., J.-P.W. and J.W. wrote the paper. J.W. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary References, Supplementary Figure legends and Supplementary Figures 1-12, Supplementary Table titles 1-3 and Supplementary Table 1 (see separate files for Supplementary Tables 2-3). (PDF 5030 kb)
Supplementary Table 2
This file contains a list of nucleosomes in the unique map with NCP score and NCP score/noise ratio. Tab delimited text file. The names of the four columns are: chromosome ID, position, NCP score and NCP score/noise ratio. This file was replaced online on 21 March 2013. (TXT 2638 kb)
Supplementary Table 3
This file contains a list of nucleosomes in the redundant map with NCP score and NCP score/noise ratio. Tab delimited text file. The names of the four columns are: chromosome ID, position, NCP score and NCP score/noise ratio. This file was replaced online on 21 March 2013. (TXT 13465 kb)
Rights and permissions
About this article
Cite this article
Brogaard, K., Xi, L., Wang, JP. et al. A map of nucleosome positions in yeast at base-pair resolution. Nature 486, 496–501 (2012). https://doi.org/10.1038/nature11142
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11142
This article is cited by
-
Chromatin remodeling by Pol II primes efficient Pol III transcription
Nature Communications (2023)
-
Deciphering the mechanical code of the genome and epigenome
Nature Structural & Molecular Biology (2022)
-
Chemical map-based prediction of nucleosome positioning using the Bioconductor package nuCpos
BMC Bioinformatics (2021)
-
Measuring DNA mechanics on the genome scale
Nature (2021)
-
Multiple roles of H2A.Z in regulating promoter chromatin architecture in human cells
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.