Abstract
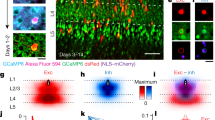
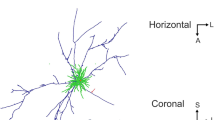
A fundamental feature of the mammalian neocortex is its columnar organization1. In the visual cortex, functional columns consisting of neurons with similar orientation preferences have been characterized extensively2,3,4, but how these columns are constructed during development remains unclear5. The radial unit hypothesis6 posits that the ontogenetic columns formed by clonally related neurons migrating along the same radial glial fibre during corticogenesis7 provide the basis for functional columns in adult neocortex1. However, a direct correspondence between the ontogenetic and functional columns has not been demonstrated8. Here we show that, despite the lack of a discernible orientation map in mouse visual cortex4,9,10, sister neurons in the same radial clone exhibit similar orientation preferences. Using a retroviral vector encoding green fluorescent protein to label radial clones of excitatory neurons, and in vivo two-photon calcium imaging to measure neuronal response properties, we found that sister neurons preferred similar orientations whereas nearby non-sister neurons showed no such relationship. Interestingly, disruption of gap junction coupling by viral expression of a dominant-negative mutant of Cx26 (also known as Gjb2) or by daily administration of a gap junction blocker, carbenoxolone, during the first postnatal week greatly diminished the functional similarity between sister neurons, suggesting that the maturation of ontogenetic into functional columns requires intercellular communication through gap junctions. Together with the recent finding of preferential excitatory connections among sister neurons11, our results support the radial unit hypothesis and unify the ontogenetic and functional columns in the visual cortex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mountcastle, V. B. The columnar organization of the neocortex. Brain 120, 701–722 (1997)
Hubel, D. H. & Wiesel, T. N. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. (Lond.) 160, 106–154 (1962)
Bonhoeffer, T. & Grinvald, A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature 353, 429–431 (1991)
Ohki, K., Chung, S., Ch’ng, Y. H., Kara, P. & Reid, R. C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005)
White, L. E. & Fitzpatrick, D. Vision and cortical map development. Neuron 56, 327–338 (2007)
Rakic, P. Specification of cerebral cortical areas. Science 241, 170–176 (1988)
Noctor, S. C., Flint, A. C., Weissman, T. A., Dammerman, R. S. & Kriegstein, A. R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720 (2001)
Horton, J. C. & Adams, D. L. The cortical column: a structure without a function. Phil. Trans. R. Soc. Lond. B 360, 837–862 (2005)
Schuett, S., Bonhoeffer, T. & Hubener, M. Mapping retinotopic structure in mouse visual cortex with optical imaging. J. Neurosci. 22, 6549–6559 (2002)
Ko, H. et al. Functional specificity of local synaptic connections in neocortical networks. Nature 473, 87–91 (2011)
Yu, Y. C., Bultje, R. S., Wang, X. & Shi, S. H. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature 458, 501–504 (2009)
Cepko, C. L. et al. Studies of cortical development using retrovirus vectors. Cold Spring Harb. Symp. Quant. Biol. 55, 265–278 (1990)
Polleux, F., Dehay, C. & Kennedy, H. The timetable of laminar neurogenesis contributes to the specification of cortical areas in mouse isocortex. J. Comp. Neurol. 385, 95–116 (1997)
Denk, W., Strickler, J. H. & Webb, W. W. 2-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990)
Stosiek, C., Garaschuk, O., Holthoff, K. & Konnerth, A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl Acad. Sci. USA 100, 7319–7324 (2003)
Walsh, C. & Cepko, C. L. Clonal dispersion in proliferative layers of developing cerebral cortex. Nature 362, 632–635 (1993)
Rochefort, N. L. et al. Development of direction selectivity in mouse cortical neurons. Neuron 71, 425–432 (2011)
Yuste, R., Peinado, A. & Katz, L. C. Neuronal domains in developing neocortex. Science 257, 665–669 (1992)
Nadarajah, B., Jones, A. M., Evans, W. H. & Parnavelas, J. G. Differential expression of connexins during neocortical development and neuronal circuit formation. J. Neurosci. 17, 3096–3111 (1997)
Beahm, D. L. et al. Mutation of a conserved threonine in the third transmembrane helix of α- and β-connexins creates a dominant-negative closed gap junction channel. J. Biol. Chem. 281, 7994–8009 (2006)
Rouan, F. et al. Trans-dominant inhibition of connexin-43 by mutant connexin-26: implications for dominant connexin disorders affecting epidermal differentiation. J. Cell Sci. 114, 2105–2113 (2001)
Elias, L. A. & Kriegstein, A. R. Gap junctions: multifaceted regulators of embryonic cortical development. Trends Neurosci. 31, 243–250 (2008)
Kandler, K. & Katz, L. C. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J. Neurosci. 18, 1419–1427 (1998)
Peinado, A., Yuste, R. & Katz, L. C. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron 10, 103–114 (1993)
Song, S., Sjostrom, P. J., Reigl, M., Nelson, S. & Chklovskii, D. B. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3, e68 (2005)
Yoshimura, Y., Dantzker, J. L. & Callaway, E. M. Excitatory cortical neurons form fine-scale functional networks. Nature 433, 868–873 (2005)
Rockel, A. J., Hiorns, R. W. & Powell, T. P. The basic uniformity in structure of the neocortex. Brain 103, 221–244 (1980)
Niell, C. M. & Stryker, M. P. Highly selective receptive fields in mouse visual cortex. J. Neurosci. 28, 7520–7536 (2008)
Ohki, K. et al. Highly ordered arrangement of single neurons in orientation pinwheels. Nature 442, 925–928 (2006)
Koulakov, A. A. & Chklovskii, D. B. Orientation preference patterns in mammalian visual cortex: a wire length minimization approach. Neuron 29, 519–527 (2001)
van Praag, H. et al. Functional neurogenesis in the adult hippocampus. Nature 415, 1030–1034 (2002)
Garaschuk, O., Milos, R. I. & Konnerth, A. Targeted bulk-loading of fluorescent indicators for two-photon brain imaging in vivo. Nature Protocols 1, 380–386 (2006)
Pologruto, T., Sabatini, B. & Svoboda, K. ScanImage: Flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003)
Acknowledgements
This work was supported by National Institutes of Health (NIH) grant R01 EY018861 and NSF grant 22250400-42533 (to Y.D.), and NIH grants R01 DA024681 and R21NS072483 (to S.-H.S.). We thank L. E. White and S. D. Van Hooser for comments on the manuscript, A. Kwan and S. D. Van Hooser for help with two-photon imaging techniques and analysis and Y. Gu for help in making retroviruses.
Author information
Authors and Affiliations
Contributions
Y.L. performed the two-photon imaging experiments and data analysis. H.L., Y.L. and P.-l.C. performed in utero virus injection. S.G., H.X. and S.-H.S. provided the viral vectors. Y.L., H.L. and Y.D. designed the experiments and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-3. (PDF 391 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Lu, H., Cheng, Pl. et al. Clonally related visual cortical neurons show similar stimulus feature selectivity. Nature 486, 118–121 (2012). https://doi.org/10.1038/nature11110
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11110
This article is cited by
-
Microglia regulate sleep through calcium-dependent modulation of norepinephrine transmission
Nature Neuroscience (2024)
-
Somatic GJA4 gain-of-function mutation in orbital cavernous venous malformations
Angiogenesis (2023)
-
Patterned cPCDH expression regulates the fine organization of the neocortex
Nature (2022)
-
Tuning instability of non-columnar neurons in the salt-and-pepper whisker map in somatosensory cortex
Nature Communications (2022)
-
Preconfigured dynamics in the hippocampus are guided by embryonic birthdate and rate of neurogenesis
Nature Neuroscience (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.