Abstract
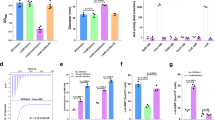
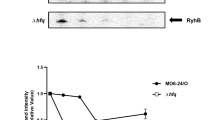
The facultative intracellular pathogen Salmonella enterica resides within a membrane-bound compartment inside macrophages1. This compartment must be acidified for Salmonella to survive within macrophages2, possibly because acidic pH promotes expression of Salmonella virulence proteins3,4. We reasoned that Salmonella might sense its surroundings have turned acidic not only upon protonation of the extracytoplasmic domain of a protein sensor5 but also by an increase in cytosolic ATP levels, because conditions that enhance the proton gradient across the bacterial inner membrane stimulate ATP synthesis6,7. Here we report that an increase in cytosolic ATP promotes transcription of the coding region for the virulence gene mgtC, which is the most highly induced horizontally acquired gene when Salmonella is inside macrophages8. This transcript is induced both upon media acidification and by physiological conditions that increase ATP levels independently of acidification. ATP is sensed by the coupling/uncoupling of transcription of the unusually long mgtC leader messenger RNA and translation of a short open reading frame located in this region. A mutation in the mgtC leader messenger RNA that eliminates the response to ATP hinders mgtC expression inside macrophages and attenuates Salmonella virulence in mice. Our results define a singular example of an ATP-sensing leader messenger RNA. Moreover, they indicate that pathogens can interpret extracellular cues by the impact they have on cellular metabolites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Garcia-del Portillo, F. Salmonella intracellular proliferation: where, when and how? Microbes Infect. 3, 1305–1311 (2001)
Rathman, M., Sjaastad, M. D. & Falkow, S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect. Immun. 64, 2765–2773 (1996)
Alpuche Aranda, C. M., Swanson, J. A., Loomis, W. P. & Miller, S. I. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc. Natl Acad. Sci. USA 89, 10079–10083 (1992)
Yu, X. J., McGourty, K., Liu, M., Unsworth, K. E. & Holden, D. W. pH sensing by intracellular Salmonella induces effector translocation. Science 328, 1040–1043 (2010)
Prost, L. R. et al. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol. Cell 26, 165–174 (2007)
Harold, F. M. & Maloney, P. C. in Escherichia coli and Salmonella: Cellular and Molecular Biology (eds Neidhart, F. C. et al.) 283–306 (American Society for Microbiology, 1996)
Senior, A. E. The proton-translocating ATPase of Escherichia coli. Annu. Rev. Biophys. Biophys. Chem. 19, 7–41 (1990)
Eriksson, S., Lucchini, S., Thompson, A., Rhen, M. & Hinton, J. C. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47, 103–118 (2003)
Blanc-Potard, A. B. & Groisman, E. A. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 16, 5376–5385 (1997)
Grabenstein, J. P., Fukuto, H. S., Palmer, L. E. & Bliska, J. B. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect. Immun. 74, 3727–3741 (2006)
Lavigne, J. P., O’Callaghan, D. & Blanc-Potard, A. B. Requirement of MgtC for Brucella suis intramacrophage growth: a potential mechanism shared by Salmonella enterica and Mycobacterium tuberculosis for adaptation to a low-Mg2+ environment. Infect. Immun. 73, 3160–3163 (2005)
Maloney, K. E. & Valvano, M. A. The mgtC gene of Burkholderia cenocepacia is required for growth under magnesium limitation conditions and intracellular survival in macrophages. Infect. Immun. 74, 5477–5486 (2006)
Buchmeier, N. et al. A parallel intraphagosomal survival strategy shared by Mycobacterium tuberculosis and Salmonella enterica. Mol. Microbiol. 35, 1375–1382 (2000)
Alix, E. & Blanc-Potard, A. B. MgtC: a key player in intramacrophage survival. Trends Microbiol. 15, 252–256 (2007)
Soncini, F. C., Garcia Vescovi, E., Solomon, F. & Groisman, E. A. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: identification of PhoP-regulated genes. J. Bacteriol. 178, 5092–5099 (1996)
Lee, E. J. & Groisman, E. A. An antisense RNA that governs the expression kinetics of a multifunctional virulence gene. Mol. Microbiol. 76, 1020–1033 (2010)
Retamal, P., Castillo-Ruiz, M. & Mora, G. C. Characterization of MgtC, a virulence factor of Salmonella enterica serovar Typhi. PLoS ONE 4, e5551 (2009)
Bearson, B. L., Wilson, L. & Foster, J. W. A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress. J. Bacteriol. 180, 2409–2417 (1998)
Chamnongpol, S. & Groisman, E. A. Acetyl phosphate-dependent activation of a mutant PhoP response regulator that functions independently of its cognate sensor kinase. J. Mol. Biol. 300, 291–305 (2000)
Turnbough, C. L., Jr & Switzer, R. L. Regulation of pyrimidine biosynthetic gene expression in bacteria: repression without repressors. Microbiol. Mol. Biol. Rev. 72, 266–300 (2008)
Merino, E. & Yanofsky, C. Transcription attenuation: a highly conserved regulatory strategy used by bacteria. Trends Genet. 21, 260–264 (2005)
Spinelli, S. V., Pontel, L. B., Garcia Vescovi, E. & Soncini, F. C. Regulation of magnesium homeostasis in Salmonella: Mg2+ targets the mgtA transcript for degradation by RNase E. FEMS Microbiol. Lett. 280, 226–234 (2008)
Amiott, E. A. & Jaehning, J. A. Mitochondrial transcription is regulated via an ATP “sensing” mechanism that couples RNA abundance to respiration. Mol. Cell 22, 329–338 (2006)
Dennis, P. B. et al. Mammalian TOR: a homeostatic ATP sensor. Science 294, 1102–1105 (2001)
Shu, D. & Guo, P. A viral RNA that binds ATP and contains a motif similar to an ATP-binding aptamer from SELEX. J. Biol. Chem. 278, 7119–7125 (2003)
Harvey, P. C. et al. Salmonella enterica serovar Typhimurium colonizing the lumen of the chicken intestine are growing slowly and up-regulate a unique set of virulence and metabolism genes. Infect. Immun. 79, 4105–4121 (2011)
Lawley, T. D. et al. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog. 2, e11 (2006)
Rehfuss, M. Y., Parker, C. T. & Brandl, M. T. Salmonella transcriptional signature in Tetrahymena phagosomes and role of acid tolerance in passage through the protist. ISME J. 5, 262–273 (2011)
Heithoff, D. M. et al. Bacterial infection as assessed by in vivo gene expression. Proc. Natl Acad. Sci. USA 94, 934–939 (1997)
Park, S. Y., Cromie, M. J., Lee, E. J. & Groisman, E. A. A bacterial mRNA leader that employs different mechanisms to sense disparate intracellular signals. Cell 142, 737–748 (2010)
Fields, P. I., Swanson, R. V., Haidaris, C. G. & Heffron, F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl Acad. Sci. USA 83, 5189–5193 (1986)
Davis, R. W., Bolstein, D. & Roth, J. R. Advanced Bacterial Genetics (Cold Spring Harbor Laboratory Press, 1980)
Snavely, M. D., Miller, C. G. & Maguire, M. E. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J. Biol. Chem. 266, 815–823 (1991)
Rust, M. J., Golden, S. S. & O’Shea, E. K. Light-driven changes in energy metabolism directly entrain the cyanobacterial circadian oscillator. Science 331, 220–223 (2011)
Jensen, K. F., Houlberg, U. & Nygaard, P. Thin-layer chromatographic methods to isolate 32P-labeled 5-phosphoribosyl-alpha-1-pyrophosphate (PRPP): determination of cellular PRPP pools and assay of PRPP synthetase activity. Anal. Biochem. 98, 254–263 (1979)
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000)
Miller, J. H. Experiments in Molecular Genetics (Cold Spring Harbor Laboratory Press, 1972)
Wilks, J. C. & Slonczewski, J. L. pH of the cytoplasm and periplasm of Escherichia coli: rapid measurement by green fluorescent protein fluorimetry. J. Bacteriol. 189, 5601–5607 (2007)
Regulski, E. E. & Breaker, R. R. In-line probing analysis of riboswitches. Methods Mol. Biol. 419, 53–67 (2008)
Acknowledgements
We thank C. Turnbough for discussions, M. Wade for help with the mouse virulence assays, and R. Breaker and A. Roth for help with in-line probing experiments. This work was supported, in part, by grant AI49561 from the National Institutes of Health to E.A.G., who is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
E.-J.L. conducted the experiments. E.-J.L. and E.A.G. designed the study and wrote the paper. Both authors read the paper and contributed to its final form.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-2, Supplementary References and Supplementary Figures 1-9. (PDF 681 kb)
Rights and permissions
About this article
Cite this article
Lee, EJ., Groisman, E. Control of a Salmonella virulence locus by an ATP-sensing leader messenger RNA. Nature 486, 271–275 (2012). https://doi.org/10.1038/nature11090
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11090
This article is cited by
-
Relationship Between Membrane Vesicles, Extracellular ATP and Biofilm Formation in Antarctic Gram-Negative Bacteria
Microbial Ecology (2021)
-
The Salmonella virulence protein MgtC promotes phosphate uptake inside macrophages
Nature Communications (2019)
-
A rule governing the FtsH-mediated proteolysis of the MgtC virulence protein from Salmonella enterica serovar Typhimurium
Journal of Microbiology (2018)
-
Elongation factor P restricts Salmonella’s growth by controlling translation of a Mg2+ transporter gene during infection
Scientific Reports (2017)
-
Non-canonical activation of OmpR drives acid and osmotic stress responses in single bacterial cells
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.