Abstract
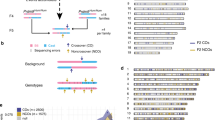
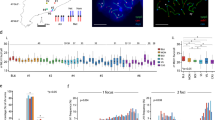
Genetic recombination occurs during meiosis, the key developmental programme of gametogenesis. Recombination in mammals has been recently linked to the activity of a histone H3 methyltransferase, PR domain containing 9 (PRDM9)1,2,3,4,5,6, the product of the only known speciation-associated gene in mammals7. PRDM9 is thought to determine the preferred recombination sites—recombination hotspots—through sequence-specific binding of its highly polymorphic multi-Zn-finger domain8. Nevertheless, Prdm9 knockout mice are proficient at initiating recombination9. Here we map and analyse the genome-wide distribution of recombination initiation sites in Prdm9 knockout mice and in two mouse strains with different Prdm9 alleles and their F1 hybrid. We show that PRDM9 determines the positions of practically all hotspots in the mouse genome, with the exception of the pseudo-autosomal region (PAR)—the only area of the genome that undergoes recombination in 100% of cells10. Surprisingly, hotspots are still observed in Prdm9 knockout mice, and as in wild type, these hotspots are found at H3 lysine 4 (H3K4) trimethylation marks. However, in the absence of PRDM9, most recombination is initiated at promoters and at other sites of PRDM9-independent H3K4 trimethylation. Such sites are rarely targeted in wild-type mice, indicating an unexpected role of the PRDM9 protein in sequestering the recombination machinery away from gene-promoter regions and other functional genomic elements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Parvanov, E. D., Petkov, P. M. & Paigen, K. Prdm9 controls activation of mammalian recombination hotspots. Science 327, 835 (2010)
Myers, S. et al. Drive against hotspot motifs in primates implicates the PRDM9 gene in meiotic recombination. Science 327, 876–879 (2010)
Baudat, F. et al. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science 327, 836–840 (2010)
Berg, I. L. et al. PRDM9 variation strongly influences recombination hot-spot activity and meiotic instability in humans. Nature Genet. 42, 859–863 (2010)
Berg, I. L. et al. Variants of the protein PRDM9 differentially regulate a set of human meiotic recombination hotspots highly active in African populations. Proc. Natl Acad. Sci. USA 108, 12378–12383 (2011)
Hinch, A. G. et al. The landscape of recombination in African–Americans. Nature 476, 170–175 (2011)
Mihola, O., Trachtulec, Z., Vlcek, C., Schimenti, J. C. & Forejt, J. A mouse speciation gene encodes a meiotic histone H3 methyltransferase. Science 323, 373–375 (2009)
Grey, C. et al. Mouse PRDM9 DNA-binding specificity determines sites of histone H3 lysine 4 trimethylation for initiation of meiotic recombination. PLoS Biol. 9, e1001176 (2011)
Hayashi, K., Yoshida, K. & Matsui, Y. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature 438, 374–378 (2005)
Burgoyne, P. S. Genetic homology and crossing over in the X and Y chromosomes of mammals. Hum. Genet. 61, 85–90 (1982)
Neale, M. J. & Keeney, S. Clarifying the mechanics of DNA strand exchange in meiotic recombination. Nature 442, 153–158 (2006)
Smagulova, F. et al. Genome-wide analysis reveals novel molecular features of mouse recombination hotspots. Nature 472, 375–378 (2011)
Khil, P. P., Smagulova, F., Brick, K. M., Camerini-Otero, R. D. & Petukhova, G. V. Sensitive mapping of recombination hotspots using sequencing-based detection of ssDNA. Genome Res. http://dx.doi.org/10.1101/gr.130583.111 (2012)
Borde, V. et al. Histone H3 lysine 4 trimethylation marks meiotic recombination initiation sites. EMBO J. 28, 99–111 (2009)
Buard, J., Barthès, P., Grey, C. & de Massy, B. Distinct histone modifications define initiation and repair of meiotic recombination in the mouse. EMBO J. 28, 2616–2624 (2009)
Guenther, M. G., Levine, S. S., Boyer, L. A., Jaenisch, R. & Young, R. A. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130, 77–88 (2007)
Ernst, J. et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473, 43–49 (2011)
Pekowska, A. et al. H3K4 tri-methylation provides an epigenetic signature of active enhancers. EMBO J. 30, 4198–4210 (2011)
Tan, M. et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 146, 1016–1028 (2011)
Pan, J. et al. A hierarchical combination of factors shapes the genome-wide topography of yeast meiotic recombination initiation. Cell 144, 719–731 (2011)
Wu, T. C. & Lichten, M. Meiosis-induced double-strand break sites determined by yeast chromatin structure. Science 263, 515–518 (1994)
Kauppi, L. et al. Distinct properties of the XY pseudoautosomal region crucial for male meiosis. Science 331, 916–920 (2011)
Oliver, P. L. et al. Accelerated evolution of the Prdm9 speciation gene across diverse metazoan taxa. PLoS Genet. 5, e1000753 (2009)
Bellott, D. W. & Page, D. C. Reconstructing the evolution of vertebrate sex chromosomes. Cold Spring Harb. Symp. Quant. Biol. 74, 345–353 (2009)
Lim, F. L., Soulez, M., Koczan, D., Thiesen, H. J. & Knight, J. C. A KRAB-related domain and a novel transcription repression domain in proteins encoded by SSX genes that are disrupted in human sarcomas. Oncogene 17, 2013–2018 (1998)
Margolin, J. F. et al. Krüppel-associated boxes are potent transcriptional repression domains. Proc. Natl Acad. Sci. USA 91, 4509–4513 (1994)
Axelsson, E. et al. Death of PRDM9 coincides with stabilization of the recombination landscape in the dog genome. Genome Res. 22, 51–63 (2012)
Muñoz-Fuentes, V., Di Rienzo, A. & Vilà, C. Prdm9, a major determinant of meiotic recombination hotspots, is not functional in dogs and their wild relatives, wolves and coyotes. PLoS ONE 6, e25498 (2011)
Jothi, R., Cuddapah, S., Barski, A., Cui, K. & Zhao, K. Genome-wide identification of in vivo protein–DNA binding sites from ChIP-Seq data. Nucleic Acids Res. 36, 5221–5231 (2008)
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008)
Acknowledgements
We thank S. Sharmeen and H. Smith for assistance with high-throughput sequencing. We also thank M. Lichten and P. Hsieh for critical discussion of the manuscript. This research was supported by the NIDDK Intramural Research Program; Basil O’Connor Starter Scholar Research Award Grant No. 5-FY07-667 from the March of Dimes Foundation (G.V.P.); NIH grant 1R01GM084104-01A1 from NIGMS (G.V.P.); and New Investigator Start-up Grants FS71HU, R071HU and CS71HU from USUHS (G.V.P.).
Author information
Authors and Affiliations
Contributions
K.B. and P.K. performed data analyses. F.S. performed all experiments. K.B. and G.V.P. wrote the manuscript. G.V.P. and R.D.C.-O. designed and supervised the study. All authors contributed to experimental design, discussed the results and critiqued the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-14, Supplementary Materials and Methods, Supplementary Table 1 and Supplementary References. (PDF 2002 kb)
Supplementary Data
This file contains a list of meiotic DSB hotspots and H3K4me3 marks in all mouse strains. (ZIP 7400 kb)
Rights and permissions
About this article
Cite this article
Brick, K., Smagulova, F., Khil, P. et al. Genetic recombination is directed away from functional genomic elements in mice. Nature 485, 642–645 (2012). https://doi.org/10.1038/nature11089
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11089
This article is cited by
-
A-MYB and BRDT-dependent RNA Polymerase II pause release orchestrates transcriptional regulation in mammalian meiosis
Nature Communications (2023)
-
Epigenetic regulation during meiosis and crossover
Physiology and Molecular Biology of Plants (2023)
-
The RNA-binding protein FUS/TLS interacts with SPO11 and PRDM9 and localize at meiotic recombination hotspots
Cellular and Molecular Life Sciences (2023)
-
Single-cell multi-omics sequencing of human spermatogenesis reveals a DNA demethylation event associated with male meiotic recombination
Nature Cell Biology (2023)
-
Analysis of archaic human haplotypes suggests that 5hmC acts as an epigenetic guide for NCO recombination
BMC Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.