Abstract
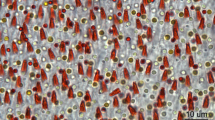
Understanding the molecular and cellular mechanisms that mediate magnetosensation in vertebrates is a formidable scientific problem1,2. One hypothesis is that magnetic information is transduced into neuronal impulses by using a magnetite-based magnetoreceptor3,4. Previous studies claim to have identified a magnetic sense system in the pigeon, common to avian species, which consists of magnetite-containing trigeminal afferents located at six specific loci in the rostral subepidermis of the beak5,6,7,8. These studies have been widely accepted in the field and heavily relied upon by both behavioural biologists and physicists9,10,11. Here we show that clusters of iron-rich cells in the rostro-medial upper beak of the pigeon Columbia livia are macrophages, not magnetosensitive neurons. Our systematic characterization of the pigeon upper beak identified iron-rich cells in the stratum laxum of the subepidermis, the basal region of the respiratory epithelium and the apex of feather follicles. Using a three-dimensional blueprint of the pigeon beak created by magnetic resonance imaging and computed tomography, we mapped the location of iron-rich cells, revealing unexpected variation in their distribution and number—an observation that is inconsistent with a role in magnetic sensation. Ultrastructure analysis of these cells, which are not unique to the beak, showed that their subcellular architecture includes ferritin-like granules, siderosomes, haemosiderin and filopodia, characteristics of iron-rich macrophages. Our conclusion that these cells are macrophages and not magnetosensitive neurons is supported by immunohistological studies showing co-localization with the antigen-presenting molecule major histocompatibility complex class II. Our work necessitates a renewed search for the true magnetite-dependent magnetoreceptor in birds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mouritsen, H. & Ritz, T. Magnetoreception and its use in bird navigation. Curr. Opin. Neurobiol. 15, 406–414 (2005)
Johnsen, S. & Lohmann, K. J. The physics and neurobiology of magnetoreception. Nature Rev. Neurosci. 6, 703–712 (2005)
Mora, C. V., Davison, M., Wild, J. M. & Walker, M. M. Magnetoreception and its trigeminal mediation in the homing pigeon. Nature 432, 508–511 (2004)
Kirschvink, J. L., Walker, M. M. & Diebel, C. E. Magnetite-based magnetoreception. Curr. Opin. Neurobiol. 11, 462–467 (2001)
Fleissner, G. et al. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J. Comp. Neurol. 458, 350–360 (2003)
Falkenberg, G. et al. Avian magnetoreception: elaborate iron mineral containing dendrites in the upper beak seem to be a common feature of birds. PLoS ONE 5, e9231 (2010)
Fleissner, G., Stahl, B., Thalau, P., Falkenberg, G. & Fleissner, G. A novel concept of Fe-mineral-based magnetoreception: histological and physicochemical data from the upper beak of homing pigeons. Naturwissenschaften 94, 631–642 (2007)
Hanzlik, M. et al. Superparamagnetic magnetite in the upper beak tissue of homing pigeons. Biometals 13, 325–331 (2000)
Wiltschko, R., Schiffner, I., Fuhrmann, P. & Wiltschko, W. The role of the magnetite-based receptors in the beak in pigeon homing. Curr. Biol. 20, 1534–1538 (2010)
Solov’yov, I. A. & Greiner, W. Theoretical analysis of an iron mineral-based magnetoreceptor model in birds. Biophys. J. 93, 1493–1509 (2007)
Davila, A. F., Winklhofer, M., Shcherbakov, V. P. & Petersen, N. Magnetic pulse affects a putative magnetoreceptor mechanism. Biophys. J. 89, 56–63 (2005)
Zapka, M. et al. Visual but not trigeminal mediation of magnetic compass information in a migratory bird. Nature 461, 1274–1277 (2009)
Ritz, T., Thalau, P., Phillips, J. B., Wiltschko, R. & Wiltschko, W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 429, 177–180 (2004)
Cadiou, H. & McNaughton, P. A. Avian magnetite-based magnetoreception: a physiologist’s perspective. J. R. Soc. Interface 7 (suppl. 2). S193–S205 (2010)
Beason, R. C. & Semm, P. Magnetic responses of the trigeminal nerve system of the bobolink (Dolichonyx oryzivorus). Neurosci. Lett. 80, 229–234 (1987)
Heyers, D., Zapka, M., Hoffmeister, M., Wild, J. M. & Mouritsen, H. Magnetic field changes activate the trigeminal brainstem complex in a migratory bird. Proc. Natl Acad. Sci. USA 107, 9394–9399 (2010)
Williams, M. N. & Wild, J. M. Trigeminally innervated iron-containing structures in the beak of homing pigeons, and other birds. Brain Res. 889, 243–246 (2001)
Wiltschko, W., Munro, U., Ford, H. & Wiltschko, R. Avian orientation: the pulse effect is mediated by the magnetite receptors in the upper beak. Proc. Biol. Sci. 276, 2227–2232 (2009)
Solov’yov, I. A. & Greiner, W. Micromagnetic insight into a magnetoreceptor in birds: existence of magnetic field amplifiers in the beak. Phys. Rev. E 80, 041919 (2009)
Stapput, K., Thalau, P., Wiltschko, R. & Wiltschko, W. Orientation of birds in total darkness. Curr. Biol. 18, 602–606 (2008)
Iancu, T. C. Ferritin and hemosiderin in pathological tissues. Electron Microsc. Rev. 5, 209–229 (1992)
Richter, G. W. The iron-loaded cell—the cytopathology of iron storage. A review. Am. J. Pathol. 91, 362–404 (1978)
Wang, J. & Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 434, 365–381 (2011)
Mebius, R. E. & Kraal, G. Structure and function of the spleen. Nature Rev. Immunol. 5, 606–616 (2005)
Meguro, R. et al. The presence of ferric and ferrous iron in the nonheme iron store of resident macrophages in different tissues and organs: histochemical demonstrations by the perfusion-Perls and -Turnbull methods in the rat. Arch. Histol. Cytol. 68, 171–183 (2005)
Simson, J. V. & Spicer, S. S. Ferritin particles in macrophages and in associated mast cells. J. Cell Biol. 52, 536–541 (1972)
Igyárto, B. Z., Lacko, E., Olah, I. & Magyar, A. Characterization of chicken epidermal dendritic cells. Immunology 119, 278–288 (2006)
Winklhofer, M. & Kirschvink, J. Does avian magnetoreception rely on both magnetite and maghemite? http://arxiv.org/abs/0805.2249 (2008)
Walker, M. M. et al. Structure and function of the vertebrate magnetic sense. Nature 390, 371–376 (1997)
Horng, Y. M., Wu, C. P., Wang, Y. C. & Huang, M. C. A novel molecular genetic marker for gender determination of pigeons. Theriogenology 65, 1759–1768 (2006)
Moos, T. & Mollgard, K. A sensitive post-DAB enhancement technique for demonstration of iron in the central nervous system. Histochemistry 99, 471–475 (1993)
Acknowledgements
We would like to thank M. Busslinger, M. Wild and J. Flint for their critical comments on earlier drafts of this manuscript. Thanks also to T. Iancu who commented on our electron micrographs and S. Soto who remarked on the inflammatory lesion in P199. Gratitude is owed to the bio-optics and electron microscopy facilities at the Institute of Molecular Pathology for their assistance in performing experiments. We wish to acknowledge the Centre for Microscopy, Characterisation and Analysis and the Australian Microscopy and Microanalysis Research Facility at the University of Western Australia, a facility funded by the University, State and Commonwealth Governments. Finally, we wish to thank Boehringer Ingelheim, which funds basic science at the Institute of Molecular Pathology.
Author information
Authors and Affiliations
Contributions
D.A.K. and C.D.T. conceived and designed the study. M.C.S., C.D.T., M.B., P.P., C.S. and N.E., performed the sectioning, PB staining and counting. D.A.K., C.D.T. and H.C. analysed the resultant data. J.R. and M.L. performed the MRI and CT studies, producing the three-dimensional structure of the pigeon beak. D.A.K. performed the immunohistochemical studies. C.D.T performed the ultrastructure experiments and J.S. and M.S. did the EFTEM and SAED studies and analysed the data. D.A.K. wrote the paper, and all authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-17 and Supplementary Tables 1-3. (PDF 9755 kb)
Supplementary Movie 1
This movie shows a three dimensional volume rendering of a pigeon head. Boundaries between skin and air or epithelium and air are shown in purple while the brain and spinal cord are indicated in yellow and the eyes in green. (MOV 7666 kb)
Supplementary Movie 2
This movie shows a series of high resolution magnetic resonance (MRI) images of a pigeon beak co-registered with computed tomography (CT). The movie starts by moving through coronal images along the rostro-caudal axis. Anatomical landmarks are indicated in red on appropriate slices (See Supplementary Figure 5). A surface rendering follows with the MRI data shown in purple, CT yellow data in yellow and landmarks in red. (MOV 8816 kb)
Rights and permissions
About this article
Cite this article
Treiber, C., Salzer, M., Riegler, J. et al. Clusters of iron-rich cells in the upper beak of pigeons are macrophages not magnetosensitive neurons. Nature 484, 367–370 (2012). https://doi.org/10.1038/nature11046
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11046
This article is cited by
-
Prussian blue technique is prone to yield false negative results in magnetoreception research
Scientific Reports (2022)
-
Investigating the impact of weak geomagnetic fluctuations on pigeon races
Journal of Comparative Physiology A (2022)
-
Antibiotics affect migratory restlessness orientation
Journal of Ethology (2022)
-
The discovery of the use of magnetic navigational information
Journal of Comparative Physiology A (2022)
-
Magnetic compass of garden warblers is not affected by oscillating magnetic fields applied to their eyes
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.