Abstract
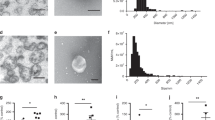
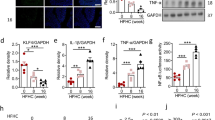
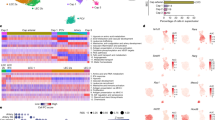
The gut microbiota is a complex ecosystem that has coevolved with host physiology. Colonization of germ-free (GF) mice with a microbiota promotes increased vessel density in the small intestine1, but little is known about the mechanisms involved. Tissue factor (TF) is the membrane receptor that initiates the extrinsic coagulation pathway2, and it promotes developmental and tumour angiogenesis3,4. Here we show that the gut microbiota promotes TF glycosylation associated with localization of TF on the cell surface, the activation of coagulation proteases, and phosphorylation of the TF cytoplasmic domain in the small intestine. Anti-TF treatment of colonized GF mice decreased microbiota-induced vascular remodelling and expression of the proangiogenic factor angiopoietin-1 (Ang-1) in the small intestine. Mice with a genetic deletion of the TF cytoplasmic domain or with hypomorphic TF (F3) alleles had a decreased intestinal vessel density. Coagulation proteases downstream of TF activate protease-activated receptor (PAR) signalling implicated in angiogenesis5. Vessel density and phosphorylation of the cytoplasmic domain of TF were decreased in small intestine from PAR1-deficient (F2r−/−) but not PAR2-deficient (F2rl1−/−) mice, and inhibition of thrombin showed that thrombin–PAR1 signalling was upstream of TF phosphorylation. Thus, the microbiota-induced extravascular TF–PAR1 signalling loop is a novel pathway that may be modulated to influence vascular remodelling in the small intestine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stappenbeck, T. S., Hooper, L. V. & Gordon, J. I. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc. Natl Acad. Sci. USA 99, 15451–15455 (2002)
Morrissey, J. H., Fakhrai, H. & Edgington, T. S. Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell 50, 129–135 (1987)
Carmeliet, P. et al. Role of tissue factor in embryonic blood vessel development. Nature 383, 73–75 (1996)
Belting, M. et al. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nature Med. 10, 502–509 (2004)
Griffin, T. C., Srinivasan, Y., Zheng, Y.-W., Huang, W. & Coughlin, S. R. A role for thrombin receptor signaling in endothelial cells during embryonic development. Science 293, 1666–1670 (2001)
Hellström, M. et al. Dll4 signalling through Notch 1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 (2007)
Sato, T. N. et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376, 70–74 (1995)
Suri, C. et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87, 1171–1180 (1996)
Iwanaga, S. The limulus clotting reaction. Curr. Opin. Immunol. 5, 74–82 (1993)
More, L. et al. Immunohistochemical study of tissue factor expression in normal intestine and idiopathic inflammatory bowel disease. J. Clin. Pathol. 46, 703–708 (1993)
Luther, T. et al. Tissue factor expression during human and mouse development. Am. J. Pathol. 149, 101–113 (1996)
Parry, G. C., Erlich, J. H., Carmeliet, P., Luther, T. & Mackman, N. Low levels of tissue factor are compatible with development and hemostasis in mice. J. Clin. Invest. 101, 560–569 (1998)
Snyder, L. A. et al. Expression of human tissue factor under the control of the mouse tissue factor promoter mediates normal hemostasis in knock-in mice. J. Thromb. Haemost. 6, 306–314 (2008)
van den Berg, Y. W. et al. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proc. Natl Acad. Sci. USA 106, 19497–19502 (2009)
Garabedian, E. M., Roberts, L. J., McNevin, M. S. & Gordon, J. I. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J. Biol. Chem. 272, 23729–23740 (1997)
Bry, L., Falk, P. G., Midtvedt, T. & Gordon, J. I. A model of host–microbial interactions in an open mammalian ecosystem. Science 273, 1380–1383 (1996)
Krudysz-Amblo, J., Jennings, M. E., II, Mann, K. G. & Butenas, S. Carbohydrates and activity of natural and recombinant tissue factor. J. Biol. Chem. 285, 3371–3382 (2010)
Camerer, E. et al. Opposite sorting of tissue factor in human umbilical vein endothelial cells and Madin–Darby canine kidney epithelial cells. Blood 88, 1339–1349 (1996)
Dorfleutner, A., Hintermann, E., Tarui, T., Takada, Y. & Ruf, W. Cross-talk of integrin α3β1 and tissue factor in cell migration. Mol. Biol. Cell 15, 4416–4425 (2004)
Zioncheck, T. F., Soumitra, R. & Vehar, G. A. The cytoplasmic domain of tissue factor is phosphorylated by a protein kinase C-dependent mechanism. J. Biol. Chem. 267, 3561–3564 (1992)
Dorfleutner, A. & Ruf, W. Regulation of tissue factor cytoplasmic domain phosphorylation by palmitoylation. Blood 102, 3998–4005 (2003)
Melis, E. et al. Targeted deletion of the cytoplasmic domain of tissue factor in mice does not affect development. Biochem. Biophys. Res. Commun. 286, 580–586 (2001)
Blackburn, J. S. & Brinckerhoff, C. E. Matrix metalloproteinase-1 and thrombin differentially activate gene expression in endothelial cells via PAR-1 and promote angiogenesis. Am. J. Pathol. 173, 1736–1746 (2008)
Schaffner, F. et al. Cooperation of tissue factor cytoplasmic domain and PAR2 signaling in breast cancer development. Blood 116, 6106–6113 (2010)
Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl Acad. Sci. USA 101, 15718–15723 (2004)
Connolly, A. J., Ishihara, H., Kahn, M. L., Farese, R. V., Jr & Coughlin, S. R. Role of the thrombin receptor in development and evidence for a second receptor. Nature 381, 516–519 (1996)
Damiano, B. P. et al. Cardiovascular responses mediated by protease-activated receptor-2 (PAR-2) and thrombin receptor (PAR-1) are distinguished in mice deficient in PAR-2 or PAR-1. J. Pharmacol. Exp. Ther. 288, 671–678 (1999)
Perreault, N. & Beaulieu, J.-F. Primary cultures of fully differentiated and pure human intestinal epithelial cells. Exp. Cell Res. 245, 34–42 (1998)
Furlan-Freguia, C., Marchese, P., Gruber, A., Ruggeri, Z. M. & Ruf, W. P2X7 receptor signaling contributes to tissue factor-dependent thrombosis in mice. J. Clin. Invest. 121, 2932–2944 (2011)
Falcón, B. L. et al. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am. J. Pathol. 175, 2159–2170 (2009)
Stappenbeck, T. S. et al. Laser capture microdissection of mouse intestine: characterizing mRNA and protein expression, and profiling intermediary metabolism in secified cell populations. Methods Enzymol. 356, 167–196 (2002)
Petersen, L. C. et al. Characterization of recombinant murine factor VIIa and recombinant murine tissue factor: a human–murine species compatibility study. Thromb. Res. 116, 75–85 (2005)
Söderberg, O. et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nature Methods 3, 995–1000 (2006)
Kirchhofer, D., Moran, P., Bullens, S. & Peale, F. A monoclonal antibody that inhibits mouse tissue factor function. J. Thromb. Haemost. 3, 1098–1099 (2005)
Acknowledgements
We thank R. Perkins for editing the manuscript; C. Arvidsson, A. Hallén, S. Wagoner, M. Karlsson, D. O’Donell, S. Islam, N. Hörmann and A. Mohammadzadeh for technical assistance; A. Hallén for providing Supplementary Fig. 16; and P. Lindahl, J. Gordon, C. Betsholtz, M. Bergö, A. Wichmann, V. Tremaroli, M. Levin and S. Massberg for comments and suggestions. We are grateful to D. Kirchhofer for the gift of 1H1 monoclonal anti-mouse TF antibody, J. Nichols at Amgen for mL4-3, N. Mackman for the low-TF mice, M. Anderson for the human TF knock-in mice, and J. Gordon for providing CR2-tox176 mice. This study was supported by the Swedish Foundation for Strategic Research, the Swedish Research Council, Torsten and Ragnar Söderberg’s foundation, Petrus and Augusta Hedlund’s foundation, and the Swedish federal government under the LUA/ALF agreement to F.B., National Institutes of Health grants HL-60742 and HL-77753 to W.R., and a Marie Curie Fellowship, a Marie Curie Reintegration Grant from the European Union and the German Federal Ministry of Education and Research (BMBF 01EO1003) to C.R.
Author information
Authors and Affiliations
Contributions
C.R. was responsible for conception and study design, biochemical analysis of TF, analysis of vessel densities, data assembly and analysis, and writing the manuscript. M.B., T.U.G., F.S. and G.Ö.L. performed data collection, analysis and interpretation and commented on the manuscript. L.C.P. provided material. W.R. and F.B. were responsible for conception and study design, data analysis and interpretation, and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-17 and Supplementary Table 1. (PDF 2185 kb)
Supplementary Movie 1
This movie shows a three dimensional rendering of a small intestinal epithelial cell stained for tissue factor (red), cytokeratin (green), and nuclei (blue). (MOV 5 kb)
Rights and permissions
About this article
Cite this article
Reinhardt, C., Bergentall, M., Greiner, T. et al. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature 483, 627–631 (2012). https://doi.org/10.1038/nature10893
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10893
This article is cited by
-
Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Comment
Internal and Emergency Medicine (2024)
-
Perinatal tissue-derived exosomes ameliorate colitis in mice by regulating the Foxp3 + Treg cells and gut microbiota
Stem Cell Research & Therapy (2023)
-
A polycistronic system for multiplexed and precalibrated expression of multigene pathways in fungi
Nature Communications (2023)
-
Commensal bacteria weaken the intestinal barrier by suppressing epithelial neuropilin-1 and Hedgehog signaling
Nature Metabolism (2023)
-
What lives on and in the sea turtle? A literature review of sea turtle bacterial microbiota
Animal Microbiome (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.