Abstract
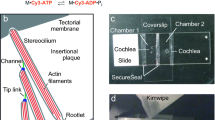
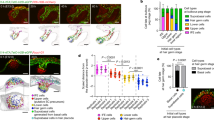
Hair cells of the inner ear are not normally replaced during an animal’s life, and must continually renew components of their various organelles1. Among these are the stereocilia, each with a core of several hundred actin filaments that arise from their apical surfaces and that bear the mechanotransduction apparatus at their tips. Actin turnover in stereocilia has previously been studied2 by transfecting neonatal rat hair cells in culture with a β-actin–GFP fusion, and evidence was found that actin is replaced, from the top down, in 2–3 days. Overexpression of the actin-binding protein espin causes elongation of stereocilia within 12–24 hours, also suggesting rapid regulation of stereocilia lengths3. Similarly, the mechanosensory ‘tip links’ are replaced in 5–10 hours after cleavage in chicken and mammalian hair cells4,5. In contrast, turnover in chick stereocilia in vivo is much slower6. It might be that only certain components of stereocilia turn over quickly, that rapid turnover occurs only in neonatal animals, only in culture, or only in response to a challenge like breakage or actin overexpression. Here we quantify protein turnover by feeding animals with a 15N-labelled precursor amino acid and using multi-isotope imaging mass spectrometry to measure appearance of new protein. Surprisingly, in adult frogs and mice and in neonatal mice, in vivo and in vitro, the stereocilia were remarkably stable, incorporating newly synthesized protein at <10% per day. Only stereocilia tips had rapid turnover and no treadmilling was observed. Other methods confirmed this: in hair cells expressing β-actin–GFP we bleached fiducial lines across hair bundles, but they did not move in 6 days. When we stopped expression of β- or γ-actin with tamoxifen-inducible recombination, neither actin isoform left the stereocilia, except at the tips. Thus, rapid turnover in stereocilia occurs only at the tips and not by a treadmilling process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schoenheimer, R. The Dynamic State of Body Constituents (Harvard Univ. Press, 1942)
Rzadzinska, A. K. et al. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J. Cell Biol. 164, 887–897 (2004)
Rzadzinska, A. et al. Balanced levels of espin are critical for stereociliary growth and length maintenance. Cell Motil. Cytoskeleton 62, 157–165 (2005)
Zhao, Y., Yamoah, E. N. & Gillespie, P. G. Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc. Natl Acad. Sci. USA 93, 15469–15474 (1996)
Jia, S., Yang, S., Guo, W. & He, D. Z. Fate of mammalian cochlear hair cells and stereocilia after loss of the stereocilia. J. Neurosci. 29, 15277–15285 (2009)
Pickles, J. O., Billieux-Hawkins, D. A. & Rouse, G. W. The incorporation and turnover of radiolabelled amino acids in developing stereocilia of the chick cochlea. Hear. Res. 101, 45–54 (1996)
Lechene, C. et al. High-resolution quantitative imaging of mammalian and bacterial cells using stable isotope mass spectrometry. J. Biol. 5, 20 (2006)
Lechene, C. P., Luyten, Y., McMahon, G. & Distel, D. L. Quantitative imaging of nitrogen fixation by individual bacteria within animal cells. Science 317, 1563–1566 (2007)
Shepherd, G. M. G., Barres, B. A. & Corey, D. P. “Bundle Blot” purification and initial protein characterization of hair-cell stereocilia. Proc. Natl Acad. Sci. USA 86, 4973–4977 (1989)
Shin, J. B. et al. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron 53, 371–386 (2007)
Schneider, M. E., Belyantseva, I. A., Azevedo, R. B. & Kachar, B. Rapid renewal of auditory hair bundles. Nature 418, 837–838 (2002)
Grati, M. et al. Rapid turnover of stereocilia membrane proteins: evidence from the trafficking and mobility of plasma membrane Ca2+-ATPase 2. J. Neurosci. 26, 6386–6395 (2006)
Kirkegaard, M. & Nyengaard, J. R. Stereological study of postnatal development in the mouse utricular macula. J. Comp. Neurol. 492, 132–144 (2005)
Perrin, B. J., Sonnemann, K. J. & Ervasti, J. M. β-actin and γ-actin are each dispensable for auditory hair cell development but required for stereocilia maintenance. PLoS Genet. 6, e1001158 (2010)
Hanft, L. M. et al. Cytoplasmic γ-actin contributes to a compensatory remodeling response in dystrophin-deficient muscle. Proc. Natl Acad. Sci. USA 103, 5385–5390 (2006)
Holt, J. R. et al. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 108, 371–381 (2002)
Prosser, H. M., Rzadzinska, A. K., Steel, K. P. & Bradley, A. Mosaic complementation demonstrates a regulatory role for myosin VIIa in actin dynamics of stereocilia. Mol. Cell. Biol. 28, 1702–1712 (2008)
Acknowledgements
C.P.L. thanks M. Raff for numerous discussions; T. Bloom for her insight, foresight and support at the origin of MIMS development. We thank D. Cotanche and G. Benichou for providing additional mouse cochlear samples, L. Trakimas for histological assistance, Z. Kaufman for assisting data analysis, and J. Hill for electronic and mechanical maintenance of the prototype instrument. G. McMahon contributed to operating the instrument and data analysis. This work was supported by National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering (NIH/NIBIB) grants P41RR14579, P41EB001974, NIH grants R01DC00033, R01DC03463, R01DC04179, R37DK39773, R01EY12963, R01GM47214 and R01D K58762, and National Science Foundation Division of Integrative Biology and Neuroscience (NSF/IBN) grant IBN-998298 to C.P.L., by NIH grant R01DC02281 to D.P.C., and by NIH grants F32DC009539 to B.J.P. and R01AR049899 to J.M.E., and by Wellcome Trust grant WT079643 to the Wellcome Trust Sanger Institute. Development of the SIMS instrument was supported by ONERA, CNRS, Université Paris Sud and Cameca (France). The work was also helped in part by software funded by the NIH National Center for Research Resources (NIH/NCRR) Center for Integrative Biomedical Computing, 2P41 RR0112553-12 and the Department of Energy SciDAC Visualization and Analytics Center for Enabling Technologies, DEFC0206ER25781. D.-S.Z. is a Research Associate, V.P. was a Research Associate and D.P.C. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
D.-S.Z. carried out the 15N experiments and the preparation of tissue for MIMS imaging. V.P. and A.K.R. conceived the photobleaching experiments; A.K.R. and H.M.P. made the β-actin–GFP mouse and V.P. did the experiments. B.J.P. and J.M.E. conceived the conditional actin deletion experiments; B.J.P. made the mice and carried out the experiments. C.P.L. and D.P.C. conceived the MIMS study of hair cells. C.P.L. developed the MIMS method, oversaw the MIMS imaging with M.W. and analysed the MIMS images with J.C.P. D.P.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-6 with legends and Supplementary References. (PDF 18421 kb)
Supplementary Movie 1
The movie shows a three-dimensional image for mass 26 (total protein) for four hair bundles of an adult mouse utricle. The imaging beam progressively etched the surface by 2-3 um, continuously acquiring images that were reconstructed into a volume image. (MOV 16450 kb)
Supplementary Movie 2
The movie shows a three dimensional image for one hair bundle of a mouse utricle showing 15N incorporation, from a neonatal mouse fed 15N for four days. The HSI scale is 0-100% incorporation in the first movie segment; then 0-45% in the second. The stereocillia have lower incorporation than do the cell bodies, but the tips of stereocillia can be seen to have higher incorporation than the stereocillia shafts. (MOV 20999 kb)
Supplementary Movie 3
The movie shows a three dimensional image for one hair bundle of a mouse cochlea showing 15N incorporation, from an adult mouse fed 15N for 56 days. The HIS scale is 0-100% incorporation. The tips of stereocillia have higher incorporation than do the stereocillia shafts; in addition, the cell membrane has higher turnover than the actin cores. (MOV 21526 kb)
Rights and permissions
About this article
Cite this article
Zhang, DS., Piazza, V., Perrin, B. et al. Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia. Nature 481, 520–524 (2012). https://doi.org/10.1038/nature10745
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10745
This article is cited by
-
Selective binding and transport of protocadherin 15 isoforms by stereocilia unconventional myosins in a heterologous expression system
Scientific Reports (2022)
-
Sample preparation optimization of insects and zebrafish for whole-body mass spectrometry imaging
Analytical and Bioanalytical Chemistry (2022)
-
Human deafness-associated variants alter the dynamics of key molecules in hair cell stereocilia F-actin cores
Human Genetics (2022)
-
Use of stable isotope-tagged thymidine and multi-isotope imaging mass spectrometry (MIMS) for quantification of human cardiomyocyte division
Nature Protocols (2021)
-
Subcellular localization of biomolecules and drug distribution by high-definition ion beam imaging
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.