Abstract
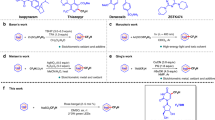
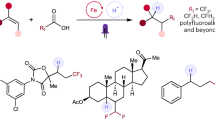
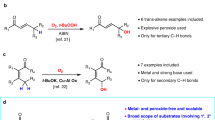
Modern drug discovery relies on the continual development of synthetic methodology to address the many challenges associated with the design of new pharmaceutical agents1. One such challenge arises from the enzymatic metabolism of drugs in vivo by cytochrome P450 oxidases, which use single-electron oxidative mechanisms to rapidly modify small molecules to facilitate their excretion2. A commonly used synthetic strategy to protect against in vivo metabolism involves the incorporation of electron-withdrawing functionality, such as the trifluoromethyl (CF3) group, into drug candidates3. The CF3 group enjoys a privileged role in the realm of medicinal chemistry because its incorporation into small molecules often enhances efficacy by promoting electrostatic interactions with targets, improving cellular membrane permeability, and increasing robustness towards oxidative metabolism of the drug4,5,6. Although common pharmacophores often bear CF3 motifs in an aromatic system, access to such analogues typically requires the incorporation of the CF3 group, or a surrogate moiety, at the start of a multi-step synthetic sequence. Here we report a mild, operationally simple strategy for the direct trifluoromethylation of unactivated arenes and heteroarenes through a radical-mediated mechanism using commercial photocatalysts and a household light bulb. We demonstrate the broad utility of this transformation through addition of CF3 to a number of heteroaromatic and aromatic systems. The benefit to medicinal chemistry and applicability to late-stage drug development is also shown through examples of the direct trifluoromethylation of widely prescribed pharmaceutical agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Li J. J., Johnson, D. S., eds. Modern Drug Synthesis (Wiley, 2010)
Montellano P. R. O., ed. Cytochrome P450: Structure, Mechanism, and Biochemistry (Springer, 2005)
Filler R., Kobayashi Y., Yagupolskii L. M., eds. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications (Elsevier, 1993)
Muller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007)
Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008)
Hagmann, W. K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 51, 4359–4369 (2008)
Tomashenko, O. A. & Grushin, V. V. Aromatic trifluoromethylation with metal complexes. Chem. Rev. 111, 4475–4521 (2011)
Furuya, T., Kamlet, A. S. & Ritter, T. Catalysis for fluorination and trifluoromethylation. Nature 473, 470–477 (2011)
Oishi, M., Kondo, H. & Amii, H. Aromatic trifluoromethylation catalytic in copper. Chem. Commun. 1909–1911 (2009)
Cho, E. J. et al. The palladium-catalyzed trifluoromethylation of aryl chlorides. Science 328, 1679–1681 (2010)
Wang, X., Truesdale, L. & Yu, J. Q. Pd (II)-catalyzed ortho-trifluoromethylation of arenes using TFA as a promoter. J. Am. Chem. Soc. 132, 3648–3649 (2010)
Xu, J. et al. Copper-catalyzed trifluoromethylation of aryl boronic acids using a CF3+ reagent. Chem. Commun. 47, 4300–4302 (2011)
Nicewicz, D. A. & MacMillan, D. W. C. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science 322, 77–80 (2008)
Yoon, T. P., Ischay, M. A. & Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nature Chem. 2, 527–532 (2010)
Narayanam, J. M. R. & Stephenson, C. R. J. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 40, 102–113 (2011)
Juris, A. et al. Ru(II) polypyridine complexes: photophysics, photochemistry, electrochemistry, and chemiluminescence. Coord. Chem. Rev. 84, 85–277 (1988)
Nagib, D. A., Scott, M. E. & MacMillan, D. W. C. Enantioselective α-trifluoromethylation of aldehydes via photoredox organocatalysis. J. Am. Chem. Soc. 131, 10875–10877 (2009)
Andrieux, C. P., Gelis, L., Medebielle, M., Pinson, J. & Saveant, J.-M. Outer-sphere dissociative electron transfer to organic molecules: a source of radicals or carbanions? Direct and indirect electrochemistry of perfluoroalkyl bromides and iodides. J. Am. Chem. Soc. 112, 3509–3520 (1990)
Skarda, V. et al. Luminescent metal complexes. Part 3. Electrochemical potentials of ground and excited states of ring-substituted 2,2’-bipyridyl and 1,10-phenanthroline tris-complexes of ruthenium. J. Chem. Soc. Perkin Trans. 2 1309–1311 (1984)
Heaton, C. A., Miller, A. K. & Powell, R. L. Predicting the reactivity of fluorinated compounds with copper using semi-empirical calculations. J. Fluor. Chem. 107, 1–3 (2001)
Heaton, C. A. & Powell, R. L. Introduction of perfluoroalkyl groups — a new approach. J. Fluor. Chem. 45, 86 (1989)
Kamigata, N., Fukushima, T. & Yoshida, M. Reactions of perfluoroalkanesulfonyl chlorides with aromatic compounds catalyzed by a ruthenium (II) complex. Chem. Lett. 19, 649–650 (1990)
Kamigata, N., Ohtsuka, T., Fukushima, T., Yoshida, M. & Shimizu, T. Direct perfluoroalkylation of aromatic and heteroaromatic compounds with perfluoroalkanesulfonyl chlorides catalysed by a ruthenium(II) phosphine complex. J. Chem. Soc. Perkin Trans. 1 1339–1346 (1994)
Dolbier, W. Fluorinated free radicals. Top. Curr. Chem. 192, 97–163 (1997)
Langlois, B. R., Laurent, E. & Roidot, N. Trifluoromethylation of aromatic compounds with sodium trifluoromethanesulfinate under oxidative conditions. Tetrahedron Lett. 32, 7525–7528 (1991)
Wiehn, M. S., Vinogradova, E. V. & Togni, A. Electrophilic trifluoromethylation of arenes and N-heteroarenes using hypervalent iodine reagents. J. Fluor. Chem. 131, 951–957 (2010)
Bahtia, K. & Schuler, R. H. Oxidation of hydroxycyclohexadienyl radical by metal ions. J. Phys. Chem. 78, 2335–2338 (1974)
Dexter, D. L., Wolberg, W. H., Ansfield, F. J., Helson, L. & Heidelberger, C. The clinical pharmacology of 5-trifluoromethyl-2'-deoxyuridine. Cancer Res. 32, 247–253 (1972)
Roth, B. D. The discovery and development of atorvastatin, a potent novel hypolipidemic agent. Prog. Med. Chem. 40, 1–22 (2002)
Ji, Y. et al. Innate C–H trifluoromethylation of heterocycles. Proc. Natl Acad. Sci. USA 108, 14411–14415 (2011)
Acknowledgements
Financial support was provided by the NIH General Medical Sciences (R01 01 GM093213-01) and gifts from Merck, Amgen, Abbott and Bristol-Myers Squibb. We thank C. Kraml and N. Byrne of Lotus Separations LLC for their development of preparatory supercritical fluid chromatography (SFC) methods and for the separation of all three CF3-Lipitor analogues.
Author information
Authors and Affiliations
Contributions
D.A.N. performed and analysed experiments. D.A.N. and D.W.C.M. designed experiments to develop this reaction and probe its utility, and also prepared this manuscript. Correspondence and requests for materials should be addressed to D.W.C.M. (dmacmill@princeton.edu).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data including Supplementary Figures 1-2 (sections 1-5) and NMR Spectra (section 6) - see contents for details. (PDF 8857 kb)
Rights and permissions
About this article
Cite this article
Nagib, D., MacMillan, D. Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. Nature 480, 224–228 (2011). https://doi.org/10.1038/nature10647
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature10647
This article is cited by
-
Experimental spectroscopic and molecular docking investigations of the anticancer drugs aprepitant and capecitabine
Theoretical Chemistry Accounts (2023)
-
Synthesis of highly efficient nitrogen enrich graphene eosin-Y coupled photocatalyst that uses solar energy in trifluoromethylation of benzaldehydes
Journal of Chemical Sciences (2023)
-
Efficacy of the monocarbonyl curcumin analog C66 in the reduction of diabetes-associated cardiovascular and kidney complications
Molecular Medicine (2022)
-
The interplay of polar effects in controlling the selectivity of radical reactions
Nature Synthesis (2022)
-
Efficient access to general α-tertiary amines via water-accelerated organocatalytic multicomponent allylation
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.