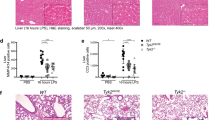
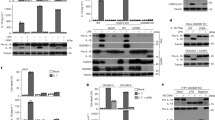
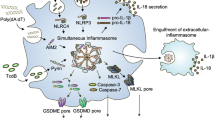
Abstract
Caspase-1 activation by inflammasome scaffolds comprised of intracellular nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and the adaptor ASC is believed to be essential for production of the pro-inflammatory cytokines interleukin (IL)-1β and IL-18 during the innate immune response1,2,3,4,5. Here we show, with C57BL/6 Casp11 gene-targeted mice, that caspase-11 (also known as caspase-4)6,7,8 is critical for caspase-1 activation and IL-1β production in macrophages infected with Escherichia coli, Citrobacter rodentium or Vibrio cholerae. Strain 129 mice, like Casp11−/− mice, exhibited defects in IL-1β production and harboured a mutation in the Casp11 locus that attenuated caspase-11 expression. This finding is important because published targeting of the Casp1 gene was done using strain 129 embryonic stem cells9,10. Casp1 and Casp11 are too close in the genome to be segregated by recombination; consequently, the published Casp1–/– mice lack both caspase-11 and caspase-1. Interestingly, Casp11–/– macrophages secreted IL-1β normally in response to ATP and monosodium urate, indicating that caspase-11 is engaged by a non-canonical inflammasome. Casp1–/–Casp11129mt/129mt macrophages expressing caspase-11 from a C57BL/6 bacterial artificial chromosome transgene failed to secrete IL-1β regardless of stimulus, confirming an essential role for caspase-1 in IL-1β production. Caspase-11 rather than caspase-1, however, was required for non-canonical inflammasome-triggered macrophage cell death, indicating that caspase-11 orchestrates both caspase-1-dependent and -independent outputs. Caspase-1 activation by non-canonical stimuli required NLRP3 and ASC, but caspase-11 processing and cell death did not, implying that there is a distinct activator of caspase-11. Lastly, loss of caspase-11 rather than caspase-1 protected mice from a lethal dose of lipopolysaccharide. These data highlight a unique pro-inflammatory role for caspase-11 in the innate immune response to clinically significant bacterial infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thornberry, N. A. et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356, 768–774 (1992)
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010)
Jin, C. & Flavell, R. A. Molecular mechanism of NLRP3 inflammasome activation. J. Clin. Immunol. 30, 628–631 (2010)
Franchi, L., Warner, N., Viani, K. & Nunez, G. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 227, 106–128 (2009)
Dinarello, C. A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117, 3720–3732 (2011)
Kang, S. J. et al. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J. Cell Biol. 149, 613–622 (2000)
Wang, S. et al. Identification and characterization of Ich-3, a member of the interleukin-1β converting enzyme (ICE)/Ced-3 family and an upstream regulator of ICE. J. Biol. Chem. 271, 20580–20587 (1996)
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998)
Kuida, K. et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science 267, 2000–2003 (1995)
Li, P. et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80, 401–411 (1995)
Freche, B., Reig, N. & van der Goot, F. G. The role of the inflammasome in cellular responses to toxins and bacterial effectors. Semin. Immunopathol. 29, 249–260 (2007)
Boyden, E. D. & Dietrich, W. F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nature Genet. 38, 240–244 (2006)
Ng, J. et al. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology 139, 542–552 (2010)
Dunne, A. et al. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis . J. Immunol. 185, 1711–1719 (2010)
Meixenberger, K. et al. Listeria monocytogenes-infected human peripheral blood mononuclear cells produce IL-1β, depending on listeriolysin O and NLRP3. J. Immunol. 184, 922–930 (2010)
Beddoe, T., Paton, A. W., Le Nours, J., Rossjohn, J. & Paton, J. C. Structure, biological functions and applications of the AB5 toxins. Trends Biochem. Sci. 35, 411–418 (2010)
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006)
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004)
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009)
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009)
Solle, M. et al. Altered cytokine production in mice lacking P2X7 receptors. J. Biol. Chem. 276, 125–132 (2001)
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006)
Toma, C. et al. Pathogenic Vibrio activate NLRP3 inflammasome via cytotoxins and TLR/nucleotide-binding oligomerization domain-mediated NF-κB signaling. J. Immunol. 184, 5287–5297 (2010)
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002)
Wickliffe, K. E., Leppla, S. H. & Moayeri, M. Anthrax lethal toxin-induced inflammasome formation and caspase-1 activation are late events dependent on ion fluxes and the proteasome. Cell. Microbiol. 10, 332–343 (2008)
Ghayur, T. et al. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature 386, 619–623 (1997)
Gu, Y. et al. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science 275, 206–209 (1997)
Walsh, J. G., Logue, S. E., Luthi, A. U. & Martin, S. J. Caspase-1 promiscuity is counterbalanced by rapid inactivation of processed enzyme. J. Biol. Chem. 286, 32513–32524 (2011)
Sutterwala, F. S. et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 (2006)
Lamkanfi, M. et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 185, 4385–4392 (2010)
Jones, J. W. et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis . Proc. Natl Acad. Sci. USA 107, 9771–9776 (2010)
Qu, Y. et al. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J. Immunol. 186, 6553–6561 (2011)
Warming, S., Costantino, N., Court, D. L., Jenkins, N. A. & Copeland, N. G. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33, e36 (2005)
Lee, E. C. et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73, 56–65 (2001)
Van Keuren, M. L., Gavrilina, G. B., Filipiak, W. E., Zeidler, M. G. & Saunders, T. L. Generating transgenic mice from bacterial artificial chromosomes: transgenesis efficiency, integration and expression outcomes. Transgenic Res. 18, 769–785 (2009)
Acknowledgements
We thank F.-X. Blaudin de Thé, A. Paler Martinez, R. J. Newman, X. Rairdan, N. Ota, J. Ngo, L. Nguyen, A. Leung, L. Tam, M. Schlatter, H. Nguyen, V. Asghari and K. O’Rourke for technical support, M. van Lookeren Campagne, D. French, S. Mariathasan, T.-D. Kanneganti and D.M. Monack for discussion and reagents.
Author information
Authors and Affiliations
Contributions
N.K., M.L., L.V.W., S.L., J.D., Y.Q. and S.H. designed and performed in vitro experiments. N.K., S.L., J.D., J.Z. and W.P.L. designed and performed in vivo experiments. S.W., M.R.-G. and K.N. generated the Casp11–/– and Casp1–/–Casp11Tg mice. J.L. performed bioinformatics analyses. N.K., S.W., K.N. and V.M.D. prepared the manuscript. N.K. and V.M.D. contributed to the study design and data analyses.
Corresponding authors
Ethics declarations
Competing interests
Most authors were employees of Genentech, Inc.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-7 with legends and Supplementary Tables 1-2. (PDF 1167 kb)
Rights and permissions
About this article
Cite this article
Kayagaki, N., Warming, S., Lamkanfi, M. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011). https://doi.org/10.1038/nature10558
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10558
This article is cited by
-
Molecular mechanism of bovine Gasdermin D-mediated pyroptosis
Veterinary Research (2024)
-
Pyroptosis: a double-edged sword in lung cancer and other respiratory diseases
Cell Communication and Signaling (2024)
-
The role of inflammasomes in human diseases and their potential as therapeutic targets
Signal Transduction and Targeted Therapy (2024)
-
Gasdermin D in macrophages drives orchitis by regulating inflammation and antigen presentation processes
EMBO Molecular Medicine (2024)
-
Crosstalk of HDAC4, PP1, and GSDMD in controlling pyroptosis
Cell Death & Disease (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.