Abstract
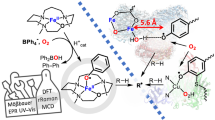
Oxygen-containing mononuclear iron species—iron(iii)–peroxo, iron(iii)–hydroperoxo and iron(iv)–oxo—are key intermediates in the catalytic activation of dioxygen by iron-containing metalloenzymes1,2,3,4,5,6,7. It has been difficult to generate synthetic analogues of these three active iron–oxygen species in identical host complexes, which is necessary to elucidate changes to the structure of the iron centre during catalysis and the factors that control their chemical reactivities with substrates. Here we report the high-resolution crystal structure of a mononuclear non-haem side-on iron(iii)–peroxo complex, [Fe(iii)(TMC)(OO)]+. We also report a series of chemical reactions in which this iron(iii)–peroxo complex is cleanly converted to the iron(iii)–hydroperoxo complex, [Fe(iii)(TMC)(OOH)]2+, via a short-lived intermediate on protonation. This iron(iii)–hydroperoxo complex then cleanly converts to the ferryl complex, [Fe(iv)(TMC)(O)]2+, via homolytic O–O bond cleavage of the iron(iii)–hydroperoxo species. All three of these iron species—the three most biologically relevant iron–oxygen intermediates—have been spectroscopically characterized; we note that they have been obtained using a simple macrocyclic ligand. We have performed relative reactivity studies on these three iron species which reveal that the iron(iii)–hydroperoxo complex is the most reactive of the three in the deformylation of aldehydes and that it has a similar reactivity to the iron(iv)–oxo complex in C–H bond activation of alkylaromatics. These reactivity results demonstrate that iron(iii)–hydroperoxo species are viable oxidants in both nucleophilic and electrophilic reactions by iron-containing enzymes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Data deposits
The crystallographic data for 1 have been deposited with the Cambridge Crystallographic Data Center under accession number CCDC 804038.
References
Solomon, E. I. et al. Geometric and electronic structure/function correlations in non-heme iron enzymes. Chem. Rev. 100, 235–350 (2000)
Kovaleva, E. G. & Lipscomb, J. D. Versatility of biological non-heme Fe(II) centers in oxygen activation reactions. Nature Chem. Biol. 4, 186–193 (2008)
Blasiak, L. C., Vaillancourt, F. H., Walsh, C. T. & Drennan, C. L. Crystal structure of the non-haem iron halogenase SyrB2 in syringomycin biosynthesis. Nature 440, 368–371 (2006)
Rittle, J. & Green, M. T. Cytochrome P450 compound I: capture, characterization, and C-H bond activation kinetics. Science 330, 933–937 (2010)
Kovaleva, E. G. & Lipscomb, J. D. Crystal structures of Fe2+ dioxygenase superoxo, alkylperoxo, and bound product intermediates. Science 316, 453–457 (2007)
Karlsson, A. et al. Crystal structure of naphthalene dioxygenase: side-on binding of dioxygen to iron. Science 299, 1039–1042 (2003)
Cicchillo, R. M. et al. An unusual carbon-carbon bond cleavage reaction during phosphinothricin biosynthesis. Nature 459, 871–874 (2009)
Seo, M. S. et al. [Mn(tmc)(O2)]+: a side-on peroxido manganese(III) complex bearing a non-heme ligand. Angew. Chem. Int. Edn 46, 377–380 (2007)
Cho, J. et al. Synthesis, structural, and spectroscopic characterization and reactivities of mononuclear cobalt(III)-peroxo complexes. J. Am. Chem. Soc. 132, 16977–16986 (2010)
Cho, J. et al. Geometric and electronic structure and reactivity of a mononuclear ‘side-on’ nickel(III)-peroxo complex. Nature Chem. 1, 568–572 (2009)
Rohde, J.-U. et al. Crystallographic and spectroscopic characterization of a nonheme Fe(IV) = O complex. Science 299, 1037–1039 (2003)
Annaraj, J., Suh, Y., Seo, M. S., Kim, S. O. & Nam, W. Mononuclear nonheme ferric-peroxo complex in aldehyde deformylation. Chem. Commun. 4529–4531 (2005)
Cramer, C. J., Tolman, W. B., Theopold, K. H. & Rheingold, A. L. Variable character of O-O and M-O bonding in side-on (η2) 1:1 metal complexes of O2 . Proc. Natl Acad. Sci. USA 100, 3635–3640 (2003)
Neese, F. & Solomon, E. I. Detailed spectroscopic and theoretical studies on [Fe(EDTA)(O2)]3–: electronic structure of the side-on ferric-peroxide bond and its relevance to reactivity. J. Am. Chem. Soc. 120, 12829–12848 (1998)
Liu, J.-G. et al. Spectroscopic characterization of a hydroperoxo-heme intermediate: conversion of a side-on peroxo to an end-on hydroperoxo complex. Angew. Chem. Int. Edn 48, 9262–9267 (2009)
Fukuzumi, S. et al. Crystal structure of a metal ion-bound oxoiron(IV) complex and implications for biological electron transfer. Nature Chem. 2, 756–759 (2010)
Jensen, K. B., McKenzie, C. J., Nielsen, L. P., Pedersen, J. Z. & Svendsen, H. M. Deprotonation of low-spin mononuclear iron(III)-hydroperoxide complexes give transient blue species assigned to high-spin iron(III)-peroxide complexes. Chem. Commun. 1313–1314 (1999)
Simaan, A. J., Banse, F., Girerd, J.-J., Wieghardt, K. & Bill, E. The electronic structure of non-heme iron(III)-hydroperoxo and iron(III)-peroxo model complexes studied by Mössbauer and electron paramagnetic resonance spectroscopies. Inorg. Chem. 40, 6538–6540 (2001)
Wada, A. et al. Reactivity of hydroperoxide bound to a mononuclear non-heme iron site. Inorg. Chem. 41, 616–618 (2002)
Westre, T. E. et al. A multiplet analysis of Fe K-edge 1s → 3d pre-edge features of iron complexes. J. Am. Chem. Soc. 119, 6297–6314 (1997)
Lehnert, N., Neese, F., Ho, R. Y. N., Que, L., Jr & Solomon, E. I. Electronic structure and reactivity of low-spin Fe(III)−hydroperoxo complexes: comparison to activated bleomycin. J. Am. Chem. Soc. 124, 10810–10822 (2002)
Li, F. et al. Characterization of a high-spin non-heme FeIII-OOH intermediate and its quantitative conversion to an FeIV = O complex. J. Am. Chem. Soc. 133, 7256–7259 (2011)
Shan, X. et al. X-ray absorption spectroscopic studies of high-spin nonheme (alkylperoxo)iron(III) intermediates. Inorg. Chem. 46, 8410–8417 (2007)
Wertz, D. L. & Valentine, J. S. Nucleophilicity of iron-peroxo porphyrin complexes. Struct. Bonding 97, 37–60 (2000)
Selke, M. & Valentine, J. S. Switching on the nucleophilic reactivity of a ferric porphyrin peroxo complex. J. Am. Chem. Soc. 120, 2652–2653 (1998)
Mayer, J. M. Understanding hydrogen atom transfer: from bond strengths to Marcus theory. Acc. Chem. Res. 44, 36–46 (2011)
Sastri, C. V. et al. Axial ligand tuning of a nonheme iron(IV)-oxo unit for hydrogen atom abstraction. Proc. Natl Acad. Sci. USA 104, 19181–19186 (2007)
Vaz, A. D. N., Roberts, E. S. & Coon, M. J. Olefin formation in the oxidative deformylation of aldehydes by cytochrome P-450. Mechanistic implications for catalysis by oxygen-derived peroxide. J. Am. Chem. Soc. 113, 5886–5887 (1991)
Akhtar, M., Corina, D., Miller, S., Shyadehi, A. Z. & Wright, J. N. Mechanism of the acyl-carbon cleavage and related reactions catalyzed by multifunctional P-450s: studies on cytochrome P-45017α . Biochemistry 33, 4410–4418 (1994)
Solomon, E. I., Wong, S. D., Liu, L. V., Decker, A. & Chow, M. S. Peroxo and oxo intermediates in mononuclear nonheme iron enzymes and related active sites. Curr. Opin. Chem. Biol. 13, 99–113 (2009)
Acknowledgements
The work was supported by NRF/MEST of Korea through the CRI (W.N.), the GRL (2010-00353; W.N.), the WCU (R31-2008-000-10010-0; W.N. and J.S.V.) and the 2011 KRICT OASIS Project (W.N.), by NIH grants GM 40392 (E.I.S.) and RR-001209 (K.O.H.), and by NSF grant MCB 0919027 (E.I.S.). J.J.B. acknowledges a Fellowship from NSF EAPSI (OISE-1014685) and the Warner Linfield Award from the University of Michigan. SSRL operations are funded by the Department of Energy (DOE) Office of Science and operated by Stanford University. The SSRL Structural Molecular Biology programme is supported by the DOE, Office of Biological and Environmental Research, and by the NIH, National Center for Research Resources (grant 5P41RR001209), Biomedical Technology Program.
Author information
Authors and Affiliations
Contributions
J.C., J.S.V., E.I.S. and W.N. conceived and designed the experiments. J.C., S.J., S.A.W., L.V.L., E.A.K., J.J.B., M.H.L., B.H. and K.O.H. performed the experiments and analysed the data. J.C., S.A.W., L.V.L., J.S.V., E.I.S. and W.N. co-wrote the Letter.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains a Supplementary Experimental Section, Supplementary Results and Discussion, Supplementary Acknowledgements, Supplementary References, Supplementary Tables 1-8 and Supplementary Figures 1-23 with legends (see Contents for full details). (PDF 2931 kb)
Rights and permissions
About this article
Cite this article
Cho, J., Jeon, S., Wilson, S. et al. Structure and reactivity of a mononuclear non-haem iron(III)–peroxo complex. Nature 478, 502–505 (2011). https://doi.org/10.1038/nature10535
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10535
This article is cited by
-
Isolation of a Ru(iv) side-on peroxo intermediate in the water oxidation reaction
Nature Chemistry (2021)
-
Nucleophilic reactivity of a copper(II)-hydroperoxo complex
Communications Chemistry (2019)
-
Regulating the coordination structure of single-atom Fe-NxCy catalytic sites for benzene oxidation
Nature Communications (2019)
-
Dioxygen activation by nonheme iron enzymes with the 2-His-1-carboxylate facial triad that generate high-valent oxoiron oxidants
JBIC Journal of Biological Inorganic Chemistry (2017)
-
Mono- and binuclear non-heme iron chemistry from a theoretical perspective
JBIC Journal of Biological Inorganic Chemistry (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.