Abstract
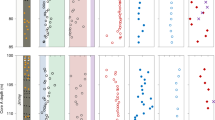
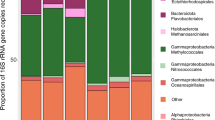
The enrichment of redox-sensitive trace metals in ancient marine sedimentary rocks has been used to determine the timing of the oxidation of the Earth’s land surface1,2. Chromium (Cr) is among the emerging proxies for tracking the effects of atmospheric oxygenation on continental weathering; this is because its supply to the oceans is dominated by terrestrial processes that can be recorded in the Cr isotope composition of Precambrian iron formations3. However, the factors controlling past and present seawater Cr isotope composition are poorly understood. Here we provide an independent and complementary record of marine Cr supply, in the form of Cr concentrations and authigenic enrichment in iron-rich sedimentary rocks. Our data suggest that Cr was largely immobile on land until around 2.48 Gyr ago, but within the 160 Myr that followed—and synchronous with independent evidence for oxygenation associated with the Great Oxidation Event (see, for example, refs 4–6)—marked excursions in Cr content and Cr/Ti ratios indicate that Cr was solubilized at a scale unrivalled in history. As Cr isotope fractionations at that time were muted, Cr must have been mobilized predominantly in reduced, Cr(iii), form. We demonstrate that only the oxidation of an abundant and previously stable crustal pyrite reservoir by aerobic-respiring, chemolithoautotrophic bacteria could have generated the degree of acidity required to solubilize Cr(iii) from ultramafic source rocks and residual soils7. This profound shift in weathering regimes beginning at 2.48 Gyr ago constitutes the earliest known geochemical evidence for acidophilic aerobes and the resulting acid rock drainage, and accounts for independent evidence of an increased supply of dissolved sulphate8 and sulphide-hosted trace elements to the oceans around that time1,9. Our model adds to amassing evidence that the Archaean-Palaeoproterozoic boundary was marked by a substantial shift in terrestrial geochemistry and biology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
07 November 2011
The scale bar in Fig. 2 appeared incorrectly in the PDF version of this paper but the print and HTML versions are correct. The PDF version has been corrected.
References
Anbar, A. D. et al. A whiff of oxygen before the Great Oxidation Event? Science 317, 1903–1906 (2007)
Wille, M. et al. Evidence for a gradual rise of oxygen between 2.6 and 2.5 Ga from Mo isotope and Re-PGE signatures in shale. Geochim. Cosmochim. Acta 71, 2417–2435 (2007)
Frei, R., Gaucher, C., Poulton, S. W. & Canfield, D. E. Fluctuations in Precambrian atmospheric oxygenation recorded by chromium isotopes. Nature 461, 250–253 (2009)
Bekker, A. et al. Dating the rise of atmospheric oxygen. Nature 427, 117–120 (2004)
Papineau, D., Mojzsis, S. J. & Schmitt, A. K. Multiple sulfur isotopes from Paleoproterozoic Huronian interglacial sediments and the rise of atmospheric oxygen. Earth Planet. Sci. Lett. 255, 188–212 (2007)
Guo, Q. et al. Reconstructing Earth’s surface oxidation across the Archean-Proterozoic transition. Geology 37, 399–402 (2009)
Rai, D., Eary, L. E. & Zachara, J. M. Environmental chemistry of chromium. Sci. Total Environ. 86, 15–23 (1989)
Reinhard, C. T. et al. A Late Archean sulfidic sea stimulated by early oxidative weathering of the continents. Science 326, 713–716 (2009)
Scott, C. et al. Tracing the stepwise oxygenation of the Proterozoic ocean. Nature 452, 456–459 (2008)
Shiraki, K. Geochemical behaviour of chromium. Resour. Geol 47, 319–330 (1997)
Oze, C., Bird, D. K. & Fendorf, S. Genesis of hexavalent chromium from natural sources in soil and groundwater. Proc. Natl Acad. Sci. USA 104, 6544–6549 (2007)
Oze, C. et al. Chromium geochemistry of serpentine soils. Int. Geol. Rev. 46, 97–126 (2004)
Wang, P.-C. et al. Isolation and characterization of an Enterobacter cloacae strain that reduces hexavalent chromium under anaerobic conditions. Appl. Environ. Microbiol. 55, 1665–1669 (1989)
Fendorf, S. E. Surface reactions of chromium in soils and waters. Geoderma 67, 55–71 (1995)
Holland, H. D. The oxygenation of the atmosphere and oceans. Phil. Trans. R. Soc. B 361, 903–915 (2006)
Lyons, T. W., Reinhard, C. T. & Scott, C. Redox redux. Geobiology 7, 489–494 (2009)
Bekker, A. et al. Iron formation: the sedimentary product of a complex interplay among mantle, tectonic, oceanic, and biospheric processes. Econ. Geol. 105, 467–508 (2010)
Spier, C. A., de Oliveira, S. M. B., Sial, A. N. & Rios, F. J. Geochemistry and genesis of the banded iron formations of the Cauê Formation, Quadrilátero Ferrífero, Minas Gerais, Brazil. Precambr. Res. 152, 170–206 (2007)
Eriksson, K. A. The Timeball Hill Formation: a fossil delta. J. Sedim. Res. 43, 1046–1053 (1973)
Morgan, J. J. Kinetics of reaction between O2 and Mn(II) species in aqueous solutions. Geochim. Cosmochim. Acta 69, 35–48 (2005)
Pavlov, A. A. & Kasting, J. F. Mass-independent fractionation of sulfur isotopes in Archean sediments: strong evidence for an anoxic Archean atmosphere. Astrobiology 2, 27–41 (2002)
Holland, H. D. Volcanic gases, black smokers, and the Great Oxidation Event. Geochim. Cosmochim. Acta 66, 3811–3826 (2002)
Moses, C. O. et al. Aqueous pyrite oxidation by dissolved oxygen and by ferric iron. Geochim. Cosmochim. Acta 51, 1561–1571 (1987)
Singer, P. C. & Stumm, W. Acidic mine drainage: the rate-determining step. Science 167, 1121–1123 (1970)
Gleisner, M., Herbert, R. B., Jr & Frogner Kockum, P. C. Pyrite oxidation by Acidithiobacillus ferrooxidans at various concentrations of dissolved oxygen. Chem. Geol. 225, 16–29 (2006)
Nordstrom, D. K. & Southam, G. in Geomicrobiology: Interactions Between Microbes and Minerals (eds Banfield, J. F. & Nealson, K. H. ) 361–390 (Vol. 35, Mineralogical Society of America, 1997)
Blank, C. E. Evolutionary timings of the origin of mesophilic sulphate reduction and oxygenic photosynthesis: a phylogenomic dating approach. Geobiology 2, 1–20 (2004)
Rye, R. & Holland, H. D. Paleosols and the evolution of atmospheric oxygen: a critical review. Am. J. Sci. 298, 621–672 (1998)
Dorland, H. C. Paleoproterozoic Laterites, Red Beds and Ironstones of the Pretoria Group with Reference to the History of Atmospheric Oxygen. M.Sc. thesis, Rand Afrikaans Univ. (1999)
Schauble, E., Rossman, G. R. & Taylor, H. P. Theoretical estimates of equilibrium chromium-isotope fractionations. Chem. Geol. 205, 99–114 (2004)
Aharon, P. Redox stratification and anoxia of the early Precambrian oceans: implications for carbon isotope excursions and oxidation events. Precambr. Res. 137, 207–222 (2005)
Condie, K. C. Chemical composition and evolution of the upper continental crust: contrasting results from surface samples and shales. Chem. Geol. 104, 1–37 (1993)
Berner, E. K. & Berner, R. A. Global Environment: Water, Air, and Geochemical Cycles (Prentice Hall, 1996)
Konhauser, K. O. et al. Oceanic nickel depletion and a methanogen famine before the Great Oxidation Event. Nature 458, 750–753 (2009)
Chester, R. Marine Geochemistry (Blackwell, 2000)
Klein, C. Some Precambrian banded iron-formations (BIFs) from around the world: their age, geologic setting, mineralogy, metamorphism, geochemistry, and origin. Am. Mineral. 90, 1473–1499 (2005)
Gustafsson, J. P. Visual Minteq. http://www.lwr.kth.se/English/OurSoftware/vminteq/ (verified, 23 August 2011)
Acknowledgements
This study was supported by the Natural Sciences and Engineering Research Council of Canada (K.O.K., S.V.L., A.B., P.W.F.), the National Science Foundation Division of Earth Sciences (T.W.L., O.J.R., L.R.K.), the National Science Foundation’s Graduate Research Program (N.J.P.), the Agouron Institute (E.P., T.W.L.), NASA’s Exobiology Program (T.W.L., S.J.M.), NASA’s Astrobiology Institute (T.W.L., L.R.K., N.J.P.), the Australian Research Council (M.E.B.), and the Conselho Nacional de Desenvolvimento Cientifico e Technológico (C.R.) We also thank V. Suckau (USIMINAS) for sample collection, G. Chen for assistance with LA-ICP-MS analyses, and J. Robbins for data compilation.
Author information
Authors and Affiliations
Contributions
Samples were provided by K.O.K., N.J.P., E.P., S.J.M., O.J.R., M.E.B., C.R., P.W.F. and A.B.; K.O.K. conceived the study; E.P. and N.J.P. performed the geochemical analyses; and S.L. conducted the data reduction and statistical analyses. K.O.K., S.V.L. and N.J.P. produced the manuscript with significant contributions from all co-authors. Specifically, insights into the geological setting for a number of samples were provided by E.P., M.E.B., C.R., P.W.F. and A.B.; use of geochemical proxies to assess Palaeoproterozoic weathering and seawater composition by T.W.L., O.J.R., L.R.K. and A.B.; and the timing and duration of the GOE by S.J.M., L.R.K. and A.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-5 with legends, a legend for Supplementary Table 1 (see separate excel file), Supplementary Table 2, a Supplementary Discussion with Supplementary Notes and Data. There are also additional references throughout. (PDF 3399 kb)
Supplementary Table 1
This table shows Cr, Ti, and Al concentrations in iron formation (BIF and GIF) through time. Also included are Phanerozoic ironstones and hydrothermal marine precipitates. (XLS 286 kb)
Rights and permissions
About this article
Cite this article
Konhauser, K., Lalonde, S., Planavsky, N. et al. Aerobic bacterial pyrite oxidation and acid rock drainage during the Great Oxidation Event. Nature 478, 369–373 (2011). https://doi.org/10.1038/nature10511
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10511
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.