Abstract
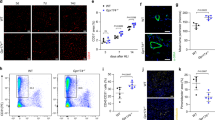
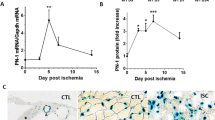
PHD2 serves as an oxygen sensor that rescues blood supply by regulating vessel formation and shape in case of oxygen shortage1,2,3,4,5. However, it is unknown whether PHD2 can influence arteriogenesis. Here we studied the role of PHD2 in collateral artery growth by using hindlimb ischaemia as a model, a process that compensates for the lack of blood flow in case of major arterial occlusion6,7,8. We show that Phd2 (also known as Egln1) haplodeficient (Phd2+/−) mice displayed preformed collateral arteries that preserved limb perfusion and prevented tissue necrosis in ischaemia. Improved arteriogenesis in Phd2+/− mice was due to an expansion of tissue-resident, M2-like macrophages9,10 and their increased release of arteriogenic factors, leading to enhanced smooth muscle cell (SMC) recruitment and growth. Both chronic and acute deletion of one Phd2 allele in macrophages was sufficient to skew their polarization towards a pro-arteriogenic phenotype. Mechanistically, collateral vessel preconditioning relied on the activation of canonical NF-κB pathway in Phd2+/− macrophages. These results unravel how PHD2 regulates arteriogenesis and artery homeostasis by controlling a specific differentiation state in macrophages and suggest new treatment options for ischaemic disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mazzone, M. et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136, 839–851 (2009)
Milkiewicz, M., Pugh, C. W. & Egginton, S. Inhibition of endogenous HIF inactivation induces angiogenesis in ischaemic skeletal muscles of mice. J. Physiol. (Lond.) 560, 21–26 (2004)
Nangaku, M. et al. A novel class of prolyl hydroxylase inhibitors induces angiogenesis and exerts organ protection against ischemia. Arterioscler. Thromb. Vasc. Biol. 27, 2548–2554 (2007)
Huang, M. et al. Short hairpin RNA interference therapy for ischemic heart disease. Circulation 118, S226–S233 (2008)
Loinard, C. et al. Inhibition of prolyl hydroxylase domain proteins promotes therapeutic revascularization. Circulation 120, 50–59 (2009)
Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nature Med. 6, 389–395 (2000)
Simons, M. & Ware, J. A. Therapeutic angiogenesis in cardiovascular disease. Nature Rev. Drug Discov. 2, 863–872 (2003)
Schaper, W. Collateral circulation: past and present. Basic Res. Cardiol. 104, 5–21 (2009)
Mantovani, A. & Sica, A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr. Opin. Immunol. 22, 231–237 (2010)
Nucera, S., Biziato, D. & De Palma, M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int. J. Dev. Biol. 55, 495–503 (2011)
Aragonés, J. et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nature Genet. 40, 170–180 (2008)
Helisch, A. et al. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler. Thromb. Vasc. Biol. 26, 520–526 (2006)
Squadrito, M. L. & De Palma, M. Macrophage regulation of tumor angiogenesis: Implications for cancer therapy. Mol. Aspects Med. 32, 123–145 (2011)
Kumar, M. S. & Owens, G. K. Combinatorial control of smooth muscle-specific gene expression. Arterioscler. Thromb. Vasc. Biol. 23, 737–747 (2003)
Wolf, C. et al. Vascular remodeling and altered protein expression during growth of coronary collateral arteries. J. Mol. Cell. Cardiol. 30, 2291–2305 (1998)
Karshovska, E., Zagorac, D., Zernecke, A., Weber, C. & Schober, A. A small molecule CXCR4 antagonist inhibits neointima formation and smooth muscle progenitor cell mobilization after arterial injury. J. Thromb. Haemost. 6, 1812–1815 (2008)
Hellstrom, M., Kalen, M., Lindahl, P., Abramsson, A. & Betsholtz, C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055 (1999)
Jaakkola, P. et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001)
Chan, D. A. et al. Tumor vasculature is regulated by PHD2-mediated angiogenesis and bone marrow-derived cell recruitment. Cancer Cell 15, 527–538 (2009)
Xue, J. et al. Prolyl hydroxylase-3 is down-regulated in colorectal cancer cells and inhibits IKKβ independent of hydroxylase activity. Gastroenterology 138, 606–615 (2010)
Cummins, E. P. et al. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proc. Natl Acad. Sci. USA 103, 18154–18159 (2006)
Fu, J. & Taubman, M. B. Prolyl hydroxylase EGLN3 regulates skeletal myoblast differentiation through an NF-κB-dependent pathway. J. Biol. Chem. 285, 8927–8935 (2010)
Mantovani, A., Garlanda, C. & Locati, M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler. Thromb. Vasc. Biol. 29, 1419–1423 (2009)
Fong, C. H. et al. An antiinflammatory role for IKKβ through the inhibition of “classical” macrophage activation. J. Exp. Med. 205, 1269–1276 (2008)
Hagemann, T. et al. “Re-educating” tumor-associated macrophages by targeting NF-κB. J. Exp. Med. 205, 1261–1268 (2008)
Greten, F. R. et al. NF-κB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell 130, 918–931 (2007)
Luttun, A. et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nature Med. 8, 831–840 (2002)
Meerpohl, H. G., Lohmann-Matthes, M. L. & Fischer, H. Studies on the activation of mouse bone marrow-derived macrophages by the macrophage cytotoxicity factor (MCF). Eur. J. Immunol. 6, 213–217 (1976)
Foo, S. S. et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124, 161–173 (2006)
Benedito, R. et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137, 1124–1135 (2009)
Chen, L. W. et al. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nature Med. 9, 575–581 (2003)
Lutgens, E. et al. Chronic myocardial infarction in the mouse: cardiac structural and functional changes. Cardiovasc. Res. 41, 586–593 (1999)
Jordan, B. F., Cron, G. O. & Gallez, B. Rapid monitoring of oxygenation by 19F magnetic resonance imaging: Simultaneous comparison with fluorescence quenching. Magn. Reson. Med. 61, 634–638 (2009)
Pfeffer, M. A. et al. Myocardial infarct size and ventricular function in rats. Circ. Res. 44, 503–512 (1979)
Acknowledgements
This work was supported by grants from FWO (G.0726.10), Belgium, and from VIB. The authors are thankful to P. Carmeliet for the Phd2 KO and cKO mice, M. Karin for the Ikbkb cKO mice, P. Ratcliffe for the PHD2H313A construct, A. Luttun and P. Fazzari for comments, Y. Jonsson and T. Janssens for technical assistance. VE-Cadherin:CreERT and PDGFRB:Cre transgenic mice were generated at Cancer Research UK and kindly donated by R. Adams. E.D. was funded by ARC, S.C. by FCT, R.L.O. and V.F. by FWO, A.H. by DFG. C.R. was supported by COST action TD0901. M.D.P. was supported by an ERC starting grant.
Author information
Authors and Affiliations
Contributions
Y.T., E.D. and S.C. performed experimental design, all experiments, acquisition of data and analysis and interpretation of all data. C.R. performed analysis of histological stainings, angiographies. R.L.O., C.R. and S.C. performed the western blots. R.L.O. and V.F. performed treadmill-running tests, quantitative PCR experiments and drug administrations. M.L.S. performed lentiviral vector preparation and cell transduction. F.B. performed EC isolation and angiography measurements. J.M., B.F.J. and B.G. performed oxymetry experiments. S.D. performed luciferase assays. M.W. and A.H. performed transplantation experiments and electroporations. Y.T. and J.S. performed the ligations of the femoral artery. Z.W.Z. and M.S. performed micro-computer tomography angiograms. A.A. and K.A. contributed vital reagents. T.B. and P.M. contributed in generating the Phd2 targeting vector. Y.T., E.D., S.C., C.R., Y.S. and M.D.P. participated in scientific discussion and drafting of the manuscript. M.M. performed experimental design, data analysis, conducted scientific direction and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-8 with legends, Supplementary Tables 1-4 and Supplementary Notes 1-6. (PDF 4149 kb)
Rights and permissions
About this article
Cite this article
Takeda, Y., Costa, S., Delamarre, E. et al. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature 479, 122–126 (2011). https://doi.org/10.1038/nature10507
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10507
This article is cited by
-
PHD1-3 oxygen sensors in vivo—lessons learned from gene deletions
Pflügers Archiv - European Journal of Physiology (2024)
-
Hypoxia-induced signaling in the cardiovascular system: pathogenesis and therapeutic targets
Signal Transduction and Targeted Therapy (2023)
-
Promoting Angiogenesis Using Immune Cells for Tissue-Engineered Vascular Grafts
Annals of Biomedical Engineering (2023)
-
p38 MAPK priming boosts VSMC proliferation and arteriogenesis by promoting PGC1α-dependent mitochondrial dynamics
Scientific Reports (2022)
-
Macrophage Polarization in Response to Biomaterials for Vascularization
Annals of Biomedical Engineering (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.