Abstract
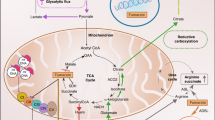
Fumarate hydratase (FH) is an enzyme of the tricarboxylic acid cycle (TCA cycle) that catalyses the hydration of fumarate into malate. Germline mutations of FH are responsible for hereditary leiomyomatosis and renal-cell cancer (HLRCC)1. It has previously been demonstrated that the absence of FH leads to the accumulation of fumarate, which activates hypoxia-inducible factors (HIFs) at normal oxygen tensions2,3,4. However, so far no mechanism that explains the ability of cells to survive without a functional TCA cycle has been provided. Here we use newly characterized genetically modified kidney mouse cells in which Fh1 has been deleted, and apply a newly developed computer model of the metabolism of these cells to predict and experimentally validate a linear metabolic pathway beginning with glutamine uptake and ending with bilirubin excretion from Fh1-deficient cells. This pathway, which involves the biosynthesis and degradation of haem, enables Fh1-deficient cells to use the accumulated TCA cycle metabolites and permits partial mitochondrial NADH production. We predicted and confirmed that targeting this pathway would render Fh1-deficient cells non-viable, while sparing wild-type Fh1-containing cells. This work goes beyond identifying a metabolic pathway that is induced in Fh1-deficient cells to demonstrate that inhibition of haem oxygenation is synthetically lethal when combined with Fh1 deficiency, providing a new potential target for treating HLRCC patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Data deposits
Microarray data are deposited at the website http://bioinformatics.picr.man.ac.uk/vice/Welcome.vice, accession reference GE_EG(2).
References
Tomlinson, I. P. et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nature Genet. 30, 406–410 (2002)
Isaacs, J. S. et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8, 143–153 (2005)
Pollard, P. J. et al. Targeted inactivation of fh1 causes proliferative renal cyst development and activation of the hypoxia pathway. Cancer Cell 11, 311–319 (2007)
Frezza, C., Pollard, P. J. & Gottlieb, E. Inborn and acquired metabolic defects in cancer. J. Mol. Med. 89, 213–220 (2011)
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA 104, 19345–19350 (2007)
Yang, Y. et al. UOK 262 cell line, fumarate hydratase deficient (FH−/FH−) hereditary leiomyomatosis renal cell carcinoma: in vitro and in vivo model of an aberrant energy metabolic pathway in human cancer. Cancer Genet. Cytogenet. 196, 45–55 (2010)
Deutscher, D., Meilijson, I., Kupiec, M. & Ruppin, E. Multiple knockout analysis of genetic robustness in the yeast metabolic network. Nature Genet. 38, 993–998 (2006)
Price, N. D., Papin, J. A., Schilling, C. H. & Palsson, B. O. Genome-scale microbial in silico models: the constraints-based approach. Trends Biotechnol. 21, 162–169 (2003)
Folger, O. et al. Predicting selective drug targets in cancer through metabolic networks. Mol. Syst. Biol. 7, 501 (2011)
Jerby, L., Shlomi, T. & Ruppin, E. Computational reconstruction of tissue-specific metabolic models: application to human liver metabolism. Mol. Syst. Biol. 6, 401 (2010)
Lee, J. K. et al. A strategy for predicting the chemosensitivity of human cancers and its application to drug discovery. Proc. Natl Acad. Sci. USA 104, 13086–13091 (2007)
Duarte, N. C. et al. Global reconstruction of the human metabolic network based on genomic and bibliomic data. Proc. Natl Acad. Sci. USA 104, 1777–1782 (2007)
Orth, J. D., Thiele, I. & Palsson, B. O. What is flux balance analysis? Nature Biotechnol. 28, 245–248 (2010)
Vanharanta, S. et al. Distinct expression profile in fumarate-hydratase-deficient uterine fibroids. Hum. Mol. Genet. 15, 97–103 (2006)
Labbe, R. F., Vreman, H. J. & Stevenson, D. K. Zinc protoporphyrin: A metabolite with a mission. Clin. Chem. 45, 2060–2072 (1999)
Schoenfeld, N. et al. The effects of metalloporphyrins, porphyrins and metals on the activity of delta-aminolevulinic acid synthase in monolayers of chick embryo liver cells. Biochem. Pharmacol. 33, 2783–2788 (1984)
Anderson, K. E. & Collins, S. Open-label study of hemin for acute porphyria: clinical practice implications. Am. J. Med. 119, e19–24 (2006)
Sudarshan, S. et al. Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1α stabilization by glucose-dependent generation of reactive oxygen species. Mol. Cell. Biol. 29, 4080–4090 (2009)
Mathew, R., Degenhardt, K., Haramaty, L., Karp, C. M. & White, E. Immortalized mouse epithelial cell models to study the role of apoptosis in cancer. Methods Enzymol. 446, 77–106 (2008)
Malena, A., Loro, E., Di Re, M., Holt, I. J. & Vergani, L. Inhibition of mitochondrial fission favours mutant over wild-type mitochondrial DNA. Hum. Mol. Genet. 18, 3407–3416 (2009)
Fernandez, C. A., Des Rosiers, C., Previs, S. F., David, F. & Brunengraber, H. Correction of 13C mass isotopomer distributions for natural stable isotope abundance. J. Mass Spectrom. 31, 255–262 (1996)
Franken, N. A., Rodermond, H. M., Stap, J., Haveman, J. & van Bree, C. Clonogenic assay of cells in vitro. Nature Protocols 1, 2315–2319 (2006)
Uc, A., Stokes, J. B. & Britigan, B. E. Heme transport exhibits polarity in Caco-2 cells: evidence for an active and membrane protein-mediated process. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G1150–G1157 (2004)
Vichai, V. & Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature Protocols 1, 1112–1116 (2006)
Acknowledgements
This work was supported by Cancer Research UK. We would like to acknowledge the Patterson Institute for Cancer Research for the Exon Array analysis. C.F. is supported by an EMBO long term fellowship (ALTF330). P.J.P. is in receipt of a Beit Memorial Fellowship funded by the Wellcome Trust (WT091112MA). D.G.W. and L.Z. would like to acknowledge SULSA (Scottish Universities Life Science Alliance). R.J.D. and K.N.R. are supported by the NIH (DK072565-05) and the Cancer Prevention and Research Institute of Texas (HIRP100437-01). O.F., T.S. and E.R. are supported by the Israel Science Foundation (ISF) and the Israel Cancer Research Foundation. L.J. is supported by the Edmond J. Safra Bioinformatics program at TAU. We thank UOB Tumor Cell Line Repository and W. Marston Linehan for providing us with the UOK262 cell lines, and A. King for editorial work.
Author information
Authors and Affiliations
Contributions
C.F. and E.G. conceived the project, analysed the data and wrote the manuscript. C.F. and. E.D.M. generated the kidney cell lines. O.F., L.J., T.S. and E.R. built the metabolic network of the cells and predicted the synthetic lethal genes. J.A., I.P.M.T. and P.J.P. provided the Fh1fl/fl mice, generated the UOKpFH cell line and provided genotyping tools. G.K. and A.H. performed the bioinformatics and statistical analysis. L.Z. and D.G.W. performed the LC–MS analysis. K.N.R. and R.J.D. performed GC–MS and the BioProfile metabolic analyses. C.F. performed all other experiments with the help of B.C. M.M. performed the electron microscopy. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-6 with legends, Supplementary Tables 5 and 6, Supplementary Text and additional references. (PDF 1467 kb)
Supplementary Table 1
This table contains the reconstructed metabolic network models for the Fh1fl/fl and Fh1-deficient cell lines. (XLS 389 kb)
Supplementary Table 2
This table contains the definition of cellular growth medium and metabolite uptake for both Fh1fl/fl and Fh1-deficient cell lines. (XLS 10 kb)
Supplementary Table 3
This table shows the reactions whose deletion is predicted to be synthetic lethal with FH. (XLS 11 kb)
Supplementary Table 4
This table contains the definition of essential biomass constituents and their relative concentration. (XLS 18 kb)
Rights and permissions
About this article
Cite this article
Frezza, C., Zheng, L., Folger, O. et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature 477, 225–228 (2011). https://doi.org/10.1038/nature10363
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10363
This article is cited by
-
Fumarate induces LncRNA-MIR4435-2HG to regulate glutamine metabolism remodeling and promote the development of FH-deficient renal cell carcinoma
Cell Death & Disease (2024)
-
Metabolic alterations in hereditary and sporadic renal cell carcinoma
Nature Reviews Nephrology (2024)
-
Bayesian kinetic modeling for tracer-based metabolomic data
BMC Bioinformatics (2023)
-
Fumarate hydratase (FH) and cancer: a paradigm of oncometabolism
British Journal of Cancer (2023)
-
Extracting functionally accurate context-specific models of Atlantic salmon metabolism
npj Systems Biology and Applications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.