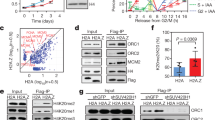
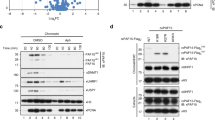
Abstract
Histone modification marks have an important role in many chromatin processes1,2. During DNA replication, both heterochromatin and euchromatin are disrupted ahead of the replication fork and are then reassembled into their original epigenetic states behind the fork3,4. How histone marks are accurately inherited from generation to generation is still poorly understood. In fission yeast (Schizosaccharomyces pombe), RNA interference (RNAi)-mediated histone methylation is cell cycle regulated. Centromeric repeats are transiently transcribed in the S phase of the cell cycle and are processed into short interfering RNAs (siRNAs) by the complexes RITS (RNA-induced initiation of transcriptional gene silencing) and RDRC (RNA-directed RNA polymerase complex)5,6,7. The small RNAs together with silencing factors—including Dos1 (also known as Clr8 and Raf1), Dos2 (also known as Clr7 and Raf2), Rik1 and Lid2—promote heterochromatic methylation of histone H3 at lysine 9 (H3K9) by a histone methyltransferase, Clr4 (refs 8–13). The methylation of H3K9 provides a binding site for Swi6, a structural and functional homologue of metazoan heterochromatin protein 1 (HP1)14. Here we characterize a silencing complex in fission yeast that contains Dos2, Rik1, Mms19 and Cdc20 (the catalytic subunit of DNA polymerase-ε). This complex regulates RNA polymerase II (RNA Pol II) activity in heterochromatin and is required for DNA replication and heterochromatin assembly. Our findings provide a molecular link between DNA replication and histone methylation, shedding light on how epigenetic marks are transmitted during each cell cycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007)
Allis, C. D., Jenuwein, T., Reinberg, D., eds. Epigenetics (Cold Spring Harbor Laboratory Press, 2006)
Probst, A. V., Dunleavy, E. & Almouzni, G. Epigenetic inheritance during the cell cycle. Nature Rev. Mol. Cell Biol. 10, 192–206 (2009)
Wallace, J. & Orr-Weaver, T. Replication of heterochromatin: insights into mechanisms of epigenetic inheritance. Chromosoma 114, 389–402 (2005)
Chen, E. S. et al. Cell cycle control of centromeric repeat transcription and heterochromatin assembly. Nature 451, 734–737 (2008)
Kloc, A., Zaratiegui, M., Nora, E. & Martienssen, R. RNA interference guides histone modification during the S phase of chromosomal replication. Curr. Biol. 18, 490–495 (2008)
Motamedi, M. R. et al. Two RNAi complexes, RITS and RDRC, physically interact and localize to noncoding centromeric RNAs. Cell 119, 789–802 (2004)
Hong, E. J., Villen, J., Gerace, E. L., Gygi, S. P. & Moazed, D. A cullin E3 ubiquitin ligase complex associates with Rik1 and the Clr4 histone H3-K9 methyltransferase and is required for RNAi-mediated heterochromatin formation. RNA Biol. 2, 106–111 (2005)
Horn, P. J., Bastie, J. N. & Peterson, C. L. A. Rik1-associated, cullin-dependent E3 ubiquitin ligase is essential for heterochromatin formation. Genes Dev. 19, 1705–1714 (2005)
Li, F. et al. Two novel proteins, Dos1 and Dos2, interact with Rik1 to regulate heterochromatic RNA interference and histone modification. Curr. Biol. 15, 1448–1457 (2005)
Li, F. et al. Lid2 is required for coordinating H3K4 and H3K9 methylation of heterochromatin and euchromatin. Cell 135, 272–283 (2008)
Thon, G. et al. The Clr7 and Clr8 directionality factors and the Pcu4 cullin mediate heterochromatin formation in the fission yeast Schizosaccharomyces pombe . Genetics 171, 1583–1595 (2005)
White, S. A. & Allshire, R. C. RNAi-mediated chromatin silencing in fission yeast. Curr. Top. Microbiol. Immunol. 320, 157–183 (2008)
Bannister, A. J. et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410, 120–124 (2001)
D’Urso, G. & Nurse, P. Schizosaccharomyces pombe cdc20+ encodes DNA polymerase ε and is required for chromosomal replication but not for the S phase checkpoint. Proc. Natl Acad. Sci. USA 94, 12491–12496 (1997)
Lauder, S. et al. Dual requirement for the yeast MMS19 gene in DNA repair and RNA polymerase II transcription. Mol. Cell. Biol. 16, 6783–6793 (1996)
Volpe, T. A. et al. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297, 1833–1837 (2002)
Hall, I. M., Noma, K. & Grewal, S. I. S. RNA interference machinery regulates chromosome dynamics during mitosis and meiosis in fission yeast. Proc. Natl Acad. Sci. USA 100, 193–198 (2003)
Jia, S. T., Noma, K. & Grewal, S. I. S. RNAi-independent heterochromatin nucleation by the stress-activated ATF/CREB family proteins. Science 304, 1971–1976 (2004)
Wu, X. Y., Li, H. & Chen, J. D. The human homologue of the yeast DNA repair and TFIIH regulator MMS19 is an AF-1-specific coactivator of estrogen receptor. J. Biol. Chem. 276, 23962–23968 (2001)
Ito, S. et al. MMXD, a TFIIH-independent XPD–MMS19 protein complex involved in chromosome segregation. Mol. Cell 39, 632–640 (2010)
Natsume, T. et al. A DNA polymerase α accessory protein, Mcl1, is required for propagation of centromere structures in fission yeast. PLoS ONE 3, e2221 (2008)
Nakayama, J., Allshire, R. C., Klar, A. J. S. & Grewal, S. I. S. Role for DNA polymerase α in epigenetic control of transcriptional silencing in fission yeast. EMBO J. 20, 2857–2866 (2001)
Feng, W. Y. & D’Urso, G. Schizosaccharomyces pombe cells lacking the amino-terminal catalytic domains of DNA polymerase ε are viable but require the DNA damage checkpoint control. Mol. Cell. Biol. 21, 4495–4504 (2001)
Kirchmaier, A. L. & Rine, J. DNA replication-independent silencing in S. cerevisiae . Science 291, 646–650 (2001)
Li, Y. C., Cheng, T. H. & Gartenberg, M. R. Establishment of transcriptional silencing in the absence of DNA replication. Science 291, 650–653 (2001)
Finnegan, E. J. & Dennis, E. S. Vernalization-induced trimethylation of histone H3 lysine 27 at FLC is not maintained in mitotically quiescent cells. Curr. Biol. 17, 1978–1983 (2007)
Yin, H. et al. Epigenetic regulation, somatic homologous recombination, and abscisic acid signaling are influenced by DNA polymerase mutation in Arabidopsis . Plant Cell 21, 386–402 (2009)
Fuss, J. & Linn, S. Human DNA polymerase ε colocalizes with proliferating cell nuclear antigen and DNA replication late, but not early, in S phase. J. Biol. Chem. 277, 8658–8666 (2002)
Moreno, S., Klar, A. & Nurse, P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe . Methods Enzymol. 194, 795–823 (1991)
Pidoux, A., Mellone, B. & Allshire, R. Analysis of chromatin in fission yeast. Methods 33, 252–259 (2004)
Smith, J. G. et al. Replication of centromere II of Schizosaccharomyces pombe . Mol. Cell. Biol. 15, 5165–5172 (1995)
Acknowledgements
We thank R. Allshire, P. Nurse, G. D’Urso and the Japan Yeast Genetic Resource Center for strains, E. Osborne and C. Hale for comments on the manuscript, Cold Spring Harbor Laboratory for mass spectrometry analysis, and members of the Cande and Li laboratories for their support and discussions. This work was supported by a grant from the National Institutes of Health (RO1GM076396) to W.Z.C. and R.M.
Author information
Authors and Affiliations
Contributions
F.L. and W.Z.C. designed the experiments and wrote the manuscript. F.L. performed the experiments. R.M. provided mass spectrometry expertise, equipment and conceptual support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-11 with legends and Supplementary Tables 1-2. (PDF 2464 kb)
Rights and permissions
About this article
Cite this article
Li, F., Martienssen, R. & Cande, W. Coordination of DNA replication and histone modification by the Rik1–Dos2 complex. Nature 475, 244–248 (2011). https://doi.org/10.1038/nature10161
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10161
This article is cited by
-
Rbm10 facilitates heterochromatin assembly via the Clr6 HDAC complex
Epigenetics & Chromatin (2021)
-
The epigenetic origin of life history transitions in plants and algae
Plant Reproduction (2021)
-
The replisome guides nucleosome assembly during DNA replication
Cell & Bioscience (2020)
-
Regulation of centromeric heterochromatin in the cell cycle by phosphorylation of histone H3 tyrosine 41
Current Genetics (2019)
-
Fe–S cluster assembly in the supergroup Excavata
JBIC Journal of Biological Inorganic Chemistry (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.