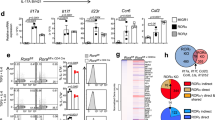
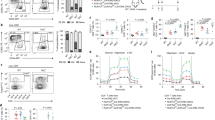
Abstract
T-helper cells that produce interleukin-17 (TH17 cells) are a recently identified CD4+ T-cell subset with characterized pathological roles in autoimmune diseases1,2,3. The nuclear receptors retinoic-acid-receptor-related orphan receptors α and γt (RORα and RORγt, respectively) have indispensible roles in the development of this cell type4,5,6,7. Here we present SR1001, a high-affinity synthetic ligand—the first in a new class of compound—that is specific to both RORα and RORγt and which inhibits TH17 cell differentiation and function. SR1001 binds specifically to the ligand-binding domains of RORα and RORγt, inducing a conformational change within the ligand-binding domain that encompasses the repositioning of helix 12 and leads to diminished affinity for co-activators and increased affinity for co-repressors, resulting in suppression of the receptors’ transcriptional activity. SR1001 inhibited the development of murine TH17 cells, as demonstrated by inhibition of interleukin-17A gene expression and protein production. Furthermore, SR1001 inhibited the expression of cytokines when added to differentiated murine or human TH17 cells. Finally, SR1001 effectively suppressed the clinical severity of autoimmune disease in mice. Our data demonstrate the feasibility of targeting the orphan receptors RORα and RORγt to inhibit specifically TH17 cell differentiation and function, and indicate that this novel class of compound has potential utility in the treatment of autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McGeachy, M. J. & Cua, D. J. Th17 cell differentiation: The long and winding road. Immunity 28, 445–453 (2008)
Bettelli, E., Korn, T., Oukka, M. & Kuchroo, V. K. Induction and effector functions of TH17 cells. Nature 453, 1051–1057 (2008)
Littman, D. R. & Rudensky, A. Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140, 845–858 (2010)
Yang, X. X. O. et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity 28, 29–39 (2008)
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006)
Ivanov, I. I., Zhou, L. & Littman, D. R. Transcriptional regulation of Th17 cell differentiation. Semin. Immunol. 19, 409–417 (2007)
Manel, N., Unutmaz, D. & Littman, D. R. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nature Immunol. 9, 641–649 (2008)
Kumar, N. et al. The benzenesulfonamide T0901317 is a novel RORα/γ inverse agonist. Mol. Pharmacol. 77, 228–236 (2010)
Zhang, F. P., Meng, G. X. & Strober, W. Interactions among the transcription factors Runx1, RORγt and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nature Immunol. 9, 1297–1306 (2008)
Ichiyama, K. et al. Foxp3 inhibits RORγt-mediated IL-17A mRNA transcription through direct interaction with RORγt. J. Biol. Chem. 283, 17003–17008 (2008)
Wang, Y. et al. Modulation of retinoic acid receptor-related orphan receptor α and γ activity by 7-oxygenated sterol ligands. J. Biol. Chem. 285, 5013–5025 (2010)
Chopra, A. R. et al. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke’s disease. Science 322, 1395–1399 (2008)
Jin, L. H. et al. Structural basis for hydroxycholesterols as natural ligands of orphan nuclear receptor RORγ. Mol. Endocrinol. 24, 923–929 (2010)
Xu, J., Wagoner, G., Douglas, J. C. & Drew, P. D. Liver X receptor agonist regulation of Th17 lympocyte function in autoimmunity. J. Leukoc. Biol. 86, 401–409 (2009)
Xu, J. H., Racke, M. K. & Drew, P. D. Peroxisome proliferator-activated receptor-α agonist fenofibrate regulates IL-12 family cytokine expression in the CNS: relevance to multiple sclerosis. J. Neurochem. 103, 1801–1810 (2007)
Delerive, P., Chin, W. W. & Suen, C. S. Identification of Reverbα as a novel RORα target gene. J. Biol. Chem. 277, 35013–35018 (2002)
Wada, T. et al. Identification of oxysterol 7α-hydroxylase (Cyp7b1) as a novel retinoid-related orphan receptor α (RORα) (NR1F1) target gene and a functional cross-talk between RORα and liver X receptor (NR1H3). Mol. Pharmacol. 73, 891–899 (2008)
Wang, J., Yin, L. & Lazar, M. A. The orphan nuclear receptor Rev-erbα regulates circadian expression of plasminogen activator inhibitor type 1. J. Biol. Chem. 281, 33842–33848 (2006)
Okamoto, K. et al. IκBζ regulates TH17 development by cooperating with ROR nuclear receptors. Nature 464, 1381–1385 (2010)
Raghuram, S. et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nature Struct. Mol. Biol. 14, 1207–1213 (2007)
Lighvani, A. A. et al. T-bet is rapidly induced by interferon-γ in lymphoid and myeloid cells. Proc. Natl Acad. Sci. USA 98, 15137–15142 (2001)
Tamauchi, H. et al. Evidence of GATA-3-dependent Th2 commitment during the in vivo immune response. Int. Immunol. 16, 179–187 (2004)
Chalmers, M. J. et al. Probing protein ligand interactions by automated hydrogen/deuterium exchange mass spectrometry. Anal. Chem. 78, 1005–1014 (2006)
Pascal, B. D., Chalmers, M. J., Busby, S. A. & Griffin, P. R. H. D. Desktop: An integrated platform for the analysis and visualization of H/D exchange data. J. Am. Soc. Mass Spectrom. 20, 601–610 (2009)
Zhang, Z. Q. & Smith, D. L. Determination of amide hydrogen-exchange by mass-spectrometry—a new tool for protein-structure elucidation. Protein Sci. 2, 522–531 (1993)
Acknowledgements
This work was supported by NIH grants to T.P.B. (DK080201, DK089984 and MH084512), to L.A.S. (DK088499) and P.R.G. (GM084041) and a grant from the National Multiple Sclerosis Society to P.D.D. (RG389A2/1). Additionally, the efforts of P.R.G. and W.R.R. were supported by the NIH Molecular Library Screening Center Network (MLSCN) grant U54MH074404 (Hugh Rosen, Principal Investigator).
Author information
Authors and Affiliations
Contributions
P.R.G. and T.P.B. conceived the project. L.A.S., P.R.G. and T.P.B. planned the project. Medicinal chemistry was planned and performed by P.N., T.M.K. and W.R.R. Biochemical and cell based assays were performed by L.A.S., N.K., Y.W., J.L. and M.A.I. Molecular modelling was performed by D.V. and S.C.C. The EAE model was designed and performed by J.X., G.W. and P.D.D. HDX studies were performed by J.L.L. The manuscript was written by L.A.S. and T.P.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
The file contains Supplementary Figures 1-9 with legends. (PDF 720 kb)
Rights and permissions
About this article
Cite this article
Solt, L., Kumar, N., Nuhant, P. et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472, 491–494 (2011). https://doi.org/10.1038/nature10075
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10075
This article is cited by
-
Targeted screening and identification of chlorhexidine as a pro-myogenic circadian clock activator
Stem Cell Research & Therapy (2023)
-
Thiostrepton alleviates experimental colitis by promoting RORγt ubiquitination and modulating dysbiosis
Cellular & Molecular Immunology (2023)
-
Chronic stress-induced depression requires the recruitment of peripheral Th17 cells into the brain
Journal of Neuroinflammation (2022)
-
Ontogeny of RORγt+ cells in the intestine of newborns and its role in the development of experimental necrotizing enterocolitis
Cell & Bioscience (2022)
-
Structural basis of synthetic agonist activation of the nuclear receptor REV-ERB
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.