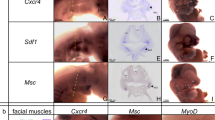
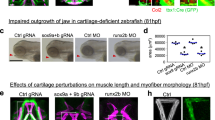
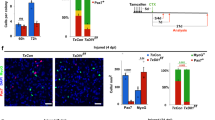
Abstract
How dynamic signalling and extensive tissue rearrangements interact to generate complex patterns and shapes during embryogenesis is poorly understood1,2,3. Here we characterize the signalling events taking place during early morphogenesis of chick skeletal muscles. We show that muscle progenitors present in somites require the transient activation of NOTCH signalling to undergo terminal differentiation. The NOTCH ligand Delta1 is expressed in a mosaic pattern in neural crest cells that migrate past the somites. Gain and loss of Delta1 function in neural crest modifies NOTCH signalling in somites, which results in delayed or premature myogenesis. Our results indicate that the neural crest regulates early muscle formation by a unique mechanism that relies on the migration of Delta1-expressing neural crest cells to trigger the transient activation of NOTCH signalling in selected muscle progenitors. This dynamic signalling guarantees a balanced and progressive differentiation of the muscle progenitor pool.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shimojo, H., Ohtsuka, T. & Kageyama, R. Oscillations in Notch signaling regulate maintenance of neural progenitors. Neuron 58, 52–64 (2008)
Joubin, K. & Stern, C. D. Molecular interactions continuously define the organizer during the cell movements of gastrulation. Cell 98, 559–571 (1999)
Palmeirim, I., Henrique, D., Ish-Horowicz, D. & Pourquie, O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell 91, 639–648 (1997)
Denetclaw, W. F., Jr, Berdougo, E., Venters, S. J. & Ordahl, C. P. Morphogenetic cell movements in the middle region of the dermomyotome dorsomedial lip associated with patterning and growth of the primary epaxial myotome. Development 128, 1745–1755 (2001)
Venters, S. J. & Ordahl, C. P. Persistent myogenic capacity of the dermomyotome dorsomedial lip and restriction of myogenic competence. Development 129, 3873–3885 (2002)
Gros, J., Scaal, M. & Marcelle, C. A two-step mechanism for myotome formation in chick. Dev. Cell 6, 875–882 (2004)
Kahane, N., Cinnamon, Y. & Kalcheim, C. The cellular mechanism by which the dermomyotome contributes to the second wave of myotome development. Development 125, 4259–4271 (1998)
Kahane, N., Cinnamon, Y. & Kalcheim, C. The roles of cell migration and myofiber intercalation in patterning formation of the postmitotic myotome. Development 129, 2675–2687 (2002)
Cinnamon, Y., Kahane, N. & Kalcheim, C. Characterization of the early development of specific hypaxial muscles from the ventrolateral myotome. Development 126, 4305–4315 (1999)
Kahane, N., Cinnamon, Y., Bachelet, I. & Kalcheim, C. The third wave of myotome colonization by mitotically competent progenitors: regulating the balance between differentiation and proliferation during muscle development. Development 128, 2187–2198 (2001)
Ben-Yair, R. & Kalcheim, C. Lineage analysis of the avian dermomyotome sheet reveals the existence of single cells with both dermal and muscle progenitor fates. Development 132, 689–701 (2005)
Gros, J., Manceau, M., Thome, V. & Marcelle, C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature 435 954–958 (2005) CrossRef
Relaix, F., Rocancourt, D., Mansouri, A. & Buckingham, M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435, 948–953 (2005)
Kassar-Duchossoy, L. et al. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 19, 1426–1431 (2005)
Hirsinger, E. et al. Notch signalling acts in postmitotic avian myogenic cells to control MyoD activation. Development 128, 107–116 (2001)
Fryer, C. J., Lamar, E., Turbachova, I., Kintner, C. & Jones, K. A. Mastermind mediates chromatin-specific transcription and turnover of the Notch enhancer complex. Genes Dev. 16, 1397–1411 (2002)
Weng, A. P. et al. Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol. Cell. Biol. 23, 655–664 (2003)
Das, R. M. et al. A robust system for RNA interference in the chicken using a modified microRNA operon. Dev. Biol. 294, 554–563 (2006)
Vasyutina, E., Lenhard, D. C. & Birchmeier, C. Notch function in myogenesis. Cell Cycle 6, 1450–1453 (2007)
Schuster-Gossler, K., Cordes, R. & Gossler, A. Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. Proc. Natl Acad. Sci. USA 104, 537–542 (2007)
Vasyutina, E. et al. RBP-J (Rbpsuh) is essential to maintain muscle progenitor cells and to generate satellite cells. Proc. Natl Acad. Sci. USA 104, 4443–4448 (2007)
De Calisto, J., Araya, C., Marchant, L., Riaz, C. F. & Mayor, R. Essential role of non-canonical Wnt signalling in neural crest migration. Development 132, 2587–2597 (2005)
Wallingford, J. B. et al. Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405, 81–85 (2000)
Rothbächer, U. et al. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 19, 1010–1022 (2000)
Gros, J., Serralbo, O. & Marcelle, C. WNT11 acts as a directional cue to organize the elongation of early muscle fibres. Nature 457, 589–593 (2009)
Henrique, D. et al. Expression of a Delta homologue in prospective neurons in the chick. Nature 375, 787–790 (1995)
Rios, A. C., Denans, N. & Marcelle, C. Real-time observation of Wnt β-catenin signaling in the chick embryo. Dev. Dyn. 239, 346–353 (2010)
Hamburger, V. & Hamilton, H. L. A series of normal stages in the development of the chick embryo. Dev. Dyn. 195, 231–272 (1992)
Ohtsuka, T. et al. Visualization of embryonic neural stem cells using Hes promoters in transgenic mice. Mol. Cell. Neurosci. 31, 109–122 (2006)
Tobiume, M. et al. Inefficient enhancement of viral infectivity and CD4 downregulation by human immunodeficiency virus type 1 Nef from Japanese long-term nonprogressors. J. Virol. 76, 5959–5965 (2002)
Daudet, N. & Lewis, J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development 132, 541–551 (2005)
Iimura, T. & Pourquie, O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature 442, 568–571 (2006)
Nagoshi, E. et al. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 119, 693–705 (2004)
Werner, T., Hammer, A., Wahlbuhl, M., Bosl, M. R. & Wegner, M. Multiple conserved regulatory elements with overlapping functions determine Sox10 expression in mouse embryogenesis. Nucleic Acids Res. 35, 6526–6538 (2007)
Uchikawa, M., Ishida, Y., Takemoto, T., Kamachi, Y. & Kondoh, H. Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Dev. Cell 4, 509–519 (2003)
Maxwell, I. H., Maxwell, F. & Glode, L. M. Regulated expression of a diphtheria toxin A-chain gene transfected into human cells: possible strategy for inducing cancer cell suicide. Cancer Res. 46, 4660–4664 (1986)
Henrique, D. et al. Maintenance of neuroepithelial progenitor cells by Delta–Notch signalling in the embryonic chick retina. Curr. Biol. 7, 661–670 (1997)
Manceau, M. et al. Myostatin promotes the terminal differentiation of embryonic muscle progenitors. Genes Dev. 22, 668–681 (2008)
Henrique, D. et al. cash4, a novel achaete-scute homolog induced by Hensen’s node during generation of the posterior nervous system. Genes Dev. 11, 603–615 (1997)
Jouve, C. et al. Notch signalling is required for cyclic expression of the hairy-like gene HES1 in the presomitic mesoderm. Development 127, 1421–1429 (2000)
Marcelle, C., Stark, M. R. & Bronner-Fraser, M. Coordinate actions of BMPs, Wnts, Shh and noggin mediate patterning of the dorsal somite. Development 124, 3955–3963 (1997)
Acknowledgements
We thank N. Rosenthal and P. Currie for critical reading of the manuscript. This study was funded by grants from the Agence Nationale pour le Recherche (ANR), and by the EU 6th Framework Programme Network of Excellence MYORES. The help of P. Weber, S. Firth, C. Johnson and I. Harper from Imaging Facilities (IBDML, Marseille and MMI, Monash University) is acknowledged.
Author information
Authors and Affiliations
Contributions
A.C.R. and C.M conceived the experiments. A.C.R. predominantly performed the work with the help of O.S. D.S. designed the animation. C.M. supervised the project and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-14 with legends. (PDF 3505 kb)
Supplementary Movie 1
This movie contains a time-lapse confocal analysis showing the rapid translocation of NOTCH-activating epithelial cells from the DML into the Transition Zone. The nuclei of the NOTCH-activating cells are in green, while all electroporated cells are in red. We estimated that it takes 90’ for a DML cell (such as the DML cell shown by a white dot) to activate NOTCH and translocate into the TZ. (ZIP 1807 kb)
Supplementary Movie 2
This movie contains a model describing the regulation of myogenesis by migrating, Delta1-expressing neural crest cells. As they migrate in close proximity to the medial border of the dermomyotome (DML, in green), Delta1-expressing neural crest establish physical contact with selected DML epithelial cells. The receiving cell transiently activates NOTCH signaling, initiates MYF5 expression (in yellow) and translocates into the Transition Zone (TZ). NOTCH signaling is repressed before TZ cells undergo terminal differentiation, elongating into myocytes and translocating into the myotome (in red). Animation created with the free open source 3D software Blender (www.blender.org). (ZIP 5988 kb)
Rights and permissions
About this article
Cite this article
Rios, A., Serralbo, O., Salgado, D. et al. Neural crest regulates myogenesis through the transient activation of NOTCH. Nature 473, 532–535 (2011). https://doi.org/10.1038/nature09970
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09970
This article is cited by
-
The Notch signaling network in muscle stem cells during development, homeostasis, and disease
Skeletal Muscle (2022)
-
MiRNA sequencing of Embryonic Myogenesis in Chengkou Mountain Chicken
BMC Genomics (2022)
-
Intramuscular delivery of neural crest stem cell spheroids enhances neuromuscular regeneration after denervation injury
Stem Cell Research & Therapy (2022)
-
TGFβ signalling acts as a molecular brake of myoblast fusion
Nature Communications (2021)
-
Vertebrate cranial mesoderm: developmental trajectory and evolutionary origin
Cellular and Molecular Life Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.