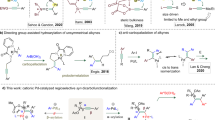
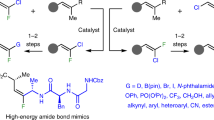
Abstract
Alkenes are found in many biologically active molecules, and there are a large number of chemical transformations in which alkenes act as the reactants or products (or both) of the reaction. Many alkenes exist as either the E or the higher-energy Z stereoisomer. Catalytic procedures for the stereoselective formation of alkenes are valuable, yet methods enabling the synthesis of 1,2-disubstituted Z alkenes are scarce. Here we report catalytic Z-selective cross-metathesis reactions of terminal enol ethers, which have not been reported previously, and of allylic amides, used until now only in E-selective processes. The corresponding disubstituted alkenes are formed in up to >98% Z selectivity and 97% yield. These transformations, promoted by catalysts that contain the highly abundant and inexpensive metal molybdenum, are amenable to gram-scale operations. Use of reduced pressure is introduced as a simple and effective strategy for achieving high stereoselectivity. The utility of this method is demonstrated by its use in syntheses of an anti-oxidant plasmalogen phospholipid, found in electrically active tissues and implicated in Alzheimer’s disease, and the potent immunostimulant KRN7000.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Negishi, E. I. et al. Recent advances in efficient and selective synthesis of di-, tri, and tetrasubstituted alkenes via Pd-catalyzed alkenylation–carbonyl olefination strategy. Acc. Chem. Res. 41, 1474–1485 (2008)
Chatterjee, A. K. in Handbook of Metathesis Vol. 1 (ed. Grubbs, R. H. ) 246–295 (Wiley-VCH, 2003)
Crowe, W. E. & Goldberg, D. R. Acrylonitrile cross-metathesis: coaxing olefin metathesis reactivity from a reluctant substrate. J. Am. Chem. Soc. 117, 5162–5163 (1995)
Hansen, E. C. & Lee, D. Efficient and Z-selective cross-metathesis of conjugated enynes. Org. Lett. 6, 2035–2038 (2004)
Malcolmson, S. J., Meek, S. J., Sattely, E. S., Schrock, R. R. & Hoveyda, A. H. Highly efficient molybdenum-based catalysts for alkene metathesis. Nature 456, 933–937 (2008)
Lee, Y.-J., Schrock, R. R. & Hoveyda, A. H. Endo-selective enyne ring-closing metathesis promoted by stereogenic-at-Mo monoalkoxide and monoaryloxide complexes. Efficient synthesis of cyclic dienes not accessible through reactions with Ru carbenes. J. Am. Chem. Soc. 131, 10652–10661 (2009)
Solans-Monfort, X., Clot, E., Copéret, C. & Eisenstein, O. d0-Re-based olefin metathesis catalysts, Re(≡CR)( = CHR)(X)(Y): the key role of X and Y ligands for efficient active sites. J. Am. Chem. Soc. 127, 14015–14025 (2005)
Meek, S. J., Malcolmson, S. J., Li, B., Schrock, R. R. & Hoveyda, A. H. The significance of degenerate processes to enantioselective olefin metathesis reactions promoted by stereogenic-at-Mo complexes. J. Am. Chem. Soc. 131, 16407–16409 (2009)
Alexander, J. B., La, D. S., Cefalo, D. R., Hoveyda, A. H. & Schrock, R. R. Catalytic enantioselective ring-closing metathesis by a chiral biphen–Mo complex. J. Am. Chem. Soc. 120, 4041–4042 (1998)
Schrock, R. R. & Hoveyda, A. H. Molybdenum and tungsten imido alkylidene complexes as efficient olefin- metathesis catalysts. Angew. Chem. Int. Edn 42, 4592–4633 (2003)
Garber, S. B., Kingsbury, J. S., Gray, B. L. & Hoveyda, A. H. Efficient and recyclable monomeric and dendritic Ru-based metathesis catalysts. J. Am. Chem. Soc. 122, 8168–8179 (2000)
Ibrahem, I., Yu, M., Schrock, R. R. & Hoveyda, A. H. Highly Z- and enantioselective ring-opening/cross-metathesis reactions catalyzed by stereogenic-at-Mo adamantylimido complexes. J. Am. Chem. Soc. 131, 3844–3845 (2009)
Jiang, A. J., Zhao, Y., Schrock, R. R. & Hoveyda, A. H. Highly Z-selective metathesis homocoupling of terminal olefins. J. Am. Chem. Soc. 131, 16630–16631 (2009)
Schrader, T. O. & Snapper, M. L. in Handbook of Metathesis (ed. Grubbs, R. H. ) 205–237 (Wiley-VCH, 2003)
La, D. S., Sattely, E. S., Ford, J. G., Schrock, R. R. & Hoveyda, A. H. Catalytic asymmetric ring-opening metathesis/cross metathesis (AROM/CM) reactions. Mechanism and application to enantioselective synthesis of functionalized cyclopentanes. J. Am. Chem. Soc. 123, 7767–7778 (2001)
Nagan, N. & Zoeller, R. A. Plasmalogens: biosynthesis and functions. Prog. Lipid Res. 40, 199–229 (2001)
Lankalapalli, R. S. et al. Synthesis and antioxidant properties of an unnatural plasmalogen analogue bearing a trans O-vinyl ether linkage. Org. Lett. 11, 2784–2787 (2009)
Lee, Y., Jang, H. & Hoveyda, A. H. Vicinal diboronates in high enantiomeric purity through tandem site-selective NHC–Cu-catalyzed boron–copper additions to terminal alkynes. J. Am. Chem. Soc. 131, 18234–18235 (2009)
Qin, D., Byun, H.-S. & Bittman, R. Synthesis of plasmalogen via 2,3-bis-O-(4’-methoxybenzyl)-sn-glycerol. J. Am. Chem. Soc. 121, 662–668 (1999)
Borg, N. A. et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 448, 44–49 (2007)
Trappeniers, M. et al. Synthesis and in vitro evaluation of α-GalCer epimers. ChemMedChem 3, 1061–1070 (2008)
Llaveria, J., Díaz, Y., Matheu, I. & Castillión, S. An efficient and general enantioselective synthesis of sphingosine, phytosphingosine, and 4-substituted derivatives. Org. Lett. 11, 205–208 (2009)
Kim, S., Song, S., Lee, T., Jung, S. & Kim, D. Practical synthesis of KRN7000 from phytosphingosine. Synthesis 847–850 (2004)
Michieletti, M. et al. Synthesis of α-galactosyl ceramide (KRN7000) and analogues thereof via a common precursor and their preliminary biological assessment. J. Org. Chem. 73, 9192–9195 (2008)
Nicolaou, K. C., Härter, M. W., Gunzner, J. L. & Nadin, A. The Wittig and related reactions in natural product synthesis. Justus Liebigs Ann. Chem. 1283–1301 (1997)
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995)
Hoveyda, A. H. & Zhugralin, A. R. The remarkable metal-catalysed olefin metathesis reaction. Nature 450, 243–251 (2007)
Hoveyda, A. H., Malcomson, S. J., Meek, S. J. & Zhugralin, A. R. Catalytic enantioselective olefin metathesis in natural product synthesis. Chiral metal-based complexes that deliver high enantioselectivity and more. Angew. Chem. Int. Edn 49, 34–44 (2010)
Nicolaou, K. C., Bulger, P. G. & Sarlah, D. Metathesis reactions in total synthesis. Angew. Chem. Int. Edn 44, 4490–4527 (2005)
Prunet, J. & Grimaud, L. in Metathesis in Natural Product Synthesis (eds Cossy, J., Arsenyadis, S. & Meyer, C. ) 287–312 (Wiley-VCH, 2010)
Acknowledgements
This research was supported by the US National Institutes of Health, Institute of General Medical Sciences (GM-59426 to A.H.H. and R.R.S.) and the National Science Foundation (CHE-0715138 to A.H.H.). R.V.O. and J.L. were LaMattina and Spanish government Visiting Scholar Fellows, respectively. We thank S. Castillón, S. J. Malcolmson and M. Yu for discussions. Mass spectrometry facilities at Boston College are supported by the US National Science Foundation (DBI-0619576).
Author information
Authors and Affiliations
Contributions
S.J.M., R.V.O. and J.L. were involved in the discovery, design and development of the new Z-selective cross-metathesis strategies and applications to the natural product syntheses. A.H.H. and R.R.S. conceived the research programme. A.H.H. directed the investigations and composed the manuscript, with revisions provided by S.J.M. and R.V.O.
Corresponding author
Ethics declarations
Competing interests
A.H.H. and R.R.S. are founders of a company that utilizes Mo-based olefin metathesis catalysts for commercial purposes.
Supplementary information
Supplementary Information
The file contains Supplementary Methods, Text and Figures (see table of contents on page 1). This file was replaced on 17 October 2011 as pages 30-92 were missing from the original file posted on line. (PDF 4549 kb)
Rights and permissions
About this article
Cite this article
Meek, S., O’Brien, R., Llaveria, J. et al. Catalytic Z-selective olefin cross-metathesis for natural product synthesis. Nature 471, 461–466 (2011). https://doi.org/10.1038/nature09957
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09957
This article is cited by
-
Bifunctional two-carbon reagent made from acetylene via 1,2-difunctionalization and its applications
Science China Chemistry (2024)
-
Chromium-catalyzed stereodivergent E- and Z-selective alkyne hydrogenation controlled by cyclic (alkyl)(amino)carbene ligands
Nature Communications (2023)
-
Effect of Lewis Acids on the Catalyst Activity for Alkene Metathesis, Z-/E- Selectivity and Stability of Tungsten Oxo Alkylidenes
Topics in Catalysis (2022)
-
Selective E to Z isomerization of 1,3-Dienes Enabled by A Dinuclear Mechanism
Nature Communications (2021)
-
Air-stable 18-electron adducts of Schrock catalysts with tuned stability constants for spontaneous release of the active species
Communications Chemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.