Abstract
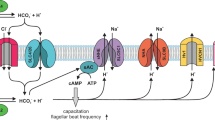
In the oviduct, cumulus cells that surround the oocyte release progesterone. In human sperm, progesterone stimulates a Ca2+ increase by a non-genomic mechanism1,2,3. The Ca2+ signal has been proposed to control chemotaxis, hyperactivation and acrosomal exocytosis of sperm4,5,6,7,8. However, the underlying signalling mechanism has remained mysterious. Here we show that progesterone activates the sperm-specific, pH-sensitive CatSper Ca2+ channel9,10,11. We found that both progesterone and alkaline pH stimulate a rapid Ca2+ influx with almost no latency, incompatible with a signalling pathway involving metabotropic receptors and second messengers. The Ca2+ signals evoked by alkaline pH and progesterone are inhibited by the Cav channel blockers NNC 55-0396 and mibefradil. Patch-clamp recordings from sperm reveal an alkaline-activated current carried by mono- and divalent ions that exhibits all the hallmarks of sperm-specific CatSper Ca2+ channels10,11. Progesterone substantially enhances the CatSper current. The alkaline- and progesterone-activated CatSper current is inhibited by both drugs. Our results resolve a long-standing controversy over the non-genomic progesterone signalling. In human sperm, either the CatSper channel itself or an associated protein serves as the non-genomic progesterone receptor. The identification of CatSper channel blockers will greatly facilitate the study of Ca2+ signalling in sperm and help to define further the physiological role of progesterone and CatSper.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Publicover, S., Harper, C. V. & Barratt, C. [Ca2+]i signalling in sperm – making the most of what you’ve got. Nature Cell Biol. 9, 235–242 (2007)
Thomas, P. & Meizel, S. Phosphatidylinositol 4,5-bisphosphate hydrolysis in human sperm stimulated with follicular fluid or progesterone is dependent upon Ca2+ influx. Biochem. J. 264, 539–546 (1989)
Blackmore, P. F., Beebe, S. J., Danforth, D. R. & Alexander, N. Progesterone and 17α-hydroxyprogesterone. Novel stimulators of calcium influx in human sperm. J. Biol. Chem. 265, 1376–1380 (1990)
Eisenbach, M. & Giojalas, L. C. Sperm guidance in mammals – an unpaved road to the egg. Nature Rev. Mol. Cell Biol. 7, 276–285 (2006)
Harper, C. V., Kirkman-Brown, J. C., Barratt, C. L. R. & Publicover, S. J. Encoding of progesterone stimulus intensity by intracellular [Ca2+] ([Ca2+]I) in human spermatozoa. Biochem. J. 373, 407–417 (2003)
Oren-Benaroya, R., Orvieto, R., Gakamsky, A., Pinchasov, M. & Eisenbach, M. The sperm chemoattractant secreted from human cumulus cells is progesterone. Hum. Reprod. 23, 2339–2345 (2008)
Suarez, S. S. Control of hyperactivation in sperm. Hum. Reprod. Update 14, 647–657 (2008)
Teves, M. E. et al. Molecular mechanism for human sperm chemotaxis mediated by progesterone. PLoS ONE 4, e8211 (2009)
Ren, D. et al. A sperm ion channel required for sperm motility and male fertility. Nature 413, 603–609 (2001)
Kirichok, Y., Navarro, B. & Clapham, D. E. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439, 737–740 (2006)
Lishko, P. V., Botchkina, I. L., Fedorenko, A. & Kirichok, Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140, 327–337 (2010)
Gellersen, B., Fernandes, M. S. & Brosens, J. J. Non-genomic progesterone actions in female reproduction. Hum. Reprod. Update 15, 119–138 (2009)
Baldi, E. et al. Nongenomic activation of spermatozoa by steroid hormones: facts and fictions. Mol. Cell. Endocrinol. 308, 39–46 (2009)
Kirkman-Brown, J. C., Bray, C., Stewart, P. M., Barratt, C. L. R. & Publicover, S. J. Biphasic elevation of [Ca2+]I in individual human spermatozoa exposed to progesterone. Dev. Biol. 222, 326–335 (2000)
Strünker, T. et al. A K+ -selective cGMP-gated ion channel controls chemosensation of sperm. Nature Cell Biol. 8, 1149–1154 (2006)
Kaupp, U. B. et al. The signal flow and motor response controlling chemotaxis of sea urchin sperm. Nature Cell Biol. 5, 109–117 (2003)
Kaupp, U. B. Olfactory signalling in vertebrates and insects: differences and commonalities. Nature Rev. Neurosci. 11, 188–200 (2010)
Blackmore, P. F., Neulen, J., Lattanzio, F. & Beebe, S. J. Cell surface-binding sites for progesterone mediate calcium uptake in human sperm. J. Biol. Chem. 266, 18655–18659 (1991)
Bedu-Addo, K. et al. Mobilisation of stored calcium in the neck region of human sperm–a mechanism for regulation of flagellar activity. Int. J. Dev. Biol. 52, 615–626 (2008)
Costello, S. et al. Ca2+-stores in sperm: their identities and functions. Reproduction 138, 425–437 (2009)
Parinaud, J. & Milhet, P. Progesterone induces Ca++-dependent 3′,5′-cyclic adenosine monophosphate increase in human sperm. J. Clin. Endocrinol. Metab. 81, 1357–1360 (1996)
Spehr, M. et al. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 299, 2054–2058 (2003)
Kamenetsky, M. et al. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J. Mol. Biol. 362, 623–639 (2006)
Carlson, A. E., Hille, B. & Babcock, D. F. External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev. Biol. 312, 183–192 (2007)
Schaefer, M., Hofmann, T., Schultz, G. & Gudermann, T. A new prostaglandin E receptor mediates calcium influx and acrosome reaction in human spermatozoa. Proc. Natl Acad. Sci. USA 95, 3008–3013 (1998)
Lishko, P. V., Botchkina, I. L. & Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature doi:10.1038/nature09767 (this issue).
Sun, F. et al. Lack of species-specificity in mammalian sperm chemotaxis. Dev. Biol. 255, 423–427 (2003)
Kaupp, U. B., Kashikar, N. D. & Weyand, I. Mechanisms of sperm chemotaxis. Annu. Rev. Physiol. 70, 93–117 (2008)
Sato, K. et al. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002–1006 (2008)
Wicher, D. et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452, 1007–1011 (2008)
Kilic, F. et al. Caged progesterone: a new tool for studying rapid nongenomic actions of progesterone. J. Am. Chem. Soc. 131, 4027–4030 (2009)
Acknowledgements
This work was supported by the German Research Foundation (SFB 645) and the International Helmholtz Research School on Biophysics and Soft Matter. We thank S. Stark for technical assistance, H. Krause and B. Kayser for preparing the manuscript, and C. Bernsdorff for preparing the figures. The caged compounds were kindly provided by V. Hagen.
Author information
Authors and Affiliations
Contributions
T.S., N.G., C.B., N.K. and I.W. designed and performed experiments. R.S. designed experiments. T.S. and U.B.K. conceived the project. T.S. and U.B.K. wrote the manuscript. All authors revised and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
The file contains Supplementary Figures 1-6 with legends. (PDF 1125 kb)
Rights and permissions
About this article
Cite this article
Strünker, T., Goodwin, N., Brenker, C. et al. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471, 382–386 (2011). https://doi.org/10.1038/nature09769
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09769
This article is cited by
-
Control of intracellular pH and bicarbonate by CO2 diffusion into human sperm
Nature Communications (2023)
-
Simulating nature in sperm selection for assisted reproduction
Nature Reviews Urology (2022)
-
Hyperactivation is sufficient to release porcine sperm from immobilized oviduct glycans
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.