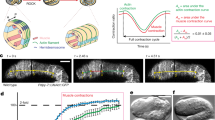
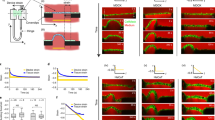
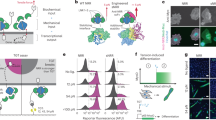
Abstract
Mechanotransduction refers to the transformation of physical forces into chemical signals. It generally involves stretch-sensitive channels or conformational change of cytoskeleton-associated proteins1. Mechanotransduction is crucial for the physiology of several organs and for cell migration2,3. The extent to which mechanical inputs contribute to development, and how they do this, remains poorly defined. Here we show that a mechanotransduction pathway operates between the body-wall muscles of Caenorhabditis elegans and the epidermis. This pathway involves, in addition to a Rac GTPase, three signalling proteins found at the hemidesmosome: p21-activated kinase (PAK-1), the adaptor GIT-1 and its partner PIX-1. The phosphorylation of intermediate filaments is one output of this pathway. Tension exerted by adjacent muscles or externally exerted mechanical pressure maintains GIT-1 at hemidesmosomes and stimulates PAK-1 activity through PIX-1 and Rac. This pathway promotes the maturation of a hemidesmosome into a junction that can resist mechanical stress and contributes to coordinating the morphogenesis of epidermal and muscle tissues. Our findings suggest that the C. elegans hemidesmosome is not only an attachment structure, but also a mechanosensor that responds to tension by triggering signalling processes. We suggest that similar pathways could promote epithelial morphogenesis or wound healing in other organisms in which epithelial cells adhere to tension-generating contractile cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Orr, A. W., Helmke, B. P., Blackman, B. R. & Schwartz, M. A. Mechanisms of mechanotransduction. Dev. Cell 10, 11–20 (2006)
Jaalouk, D. E. & Lammerding, J. Mechanotransduction gone awry. Nature Rev. Mol. Cell Biol. 10, 63–73 (2009)
Wozniak, M. A. & Chen, C. S. Mechanotransduction in development: a growing role for contractility. Nature Rev. Mol. Cell Biol. 10, 34–43 (2009)
Chisholm, A. D. & Hardin, J. Epidermal morphogenesis. in WormBook (ed. The C. elegans Research Community) doi:10.1895/wormbook.1.7.1. (2005)
Williams, B. D. & Waterston, R. H. Genes critical for muscle development and function in Caenorhabditis elegans identified through lethal mutations. J. Cell Biol. 124, 475–490 (1994)
Zhang, H. & Labouesse, M. The making of hemidesmosome structures in vivo. Dev. Dyn. 239, 1465–1476 (2010)
Costa, M., Draper, B. W. & Priess, J. R. The role of actin filaments in patterning the Caenorhabditis elegans cuticle. Dev. Biol. 184, 373–384 (1997)
Katsumi, A. et al. Effects of cell tension on the small GTPase Rac. J. Cell Biol. 158, 153–164 (2002)
Sawada, Y. & Sheetz, M. P. Force transduction by triton cytoskeletons. J. Cell Biol. 156, 609–615 (2002)
Bosher, J. M. et al. The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces. J. Cell Biol. 161, 757–768 (2003)
Hresko, M. C., Schriefer, L. A., Shrimankar, P. & Waterston, R. H. Myotactin, a novel hypodermal protein involved in muscle-cell adhesion in Caenorhabditis elegans. J. Cell Biol. 146, 659–672 (1999)
Zahreddine, H., Zhang, H., Diogon, M., Nagamatsu, Y. & Labouesse, M. CRT-1/Calreticulin and the E3 Ligase EEL-1/HUWE1 control hemidesmosome maturation in C. elegans development. Curr. Biol. 20, 322–327 (2010)
Bokoch, G. M. Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743–781 (2003)
Orr, A. W., Hahn, C., Blackman, B. R. & Schwartz, M. A. p21-activated kinase signaling regulates oxidant-dependent NF-κB activation by flow. Circ. Res. 103, 671–679 (2008)
Goto, H. et al. Phosphorylation and reorganization of vimentin by p21-activated kinase (PAK). Genes Cells 7, 91–97 (2002)
Woo, W. M., Goncharov, A., Jin, Y. & Chisholm, A. D. Intermediate filaments are required for C. elegans epidermal elongation. Dev. Biol. 267, 216–229 (2004)
Lee, R. Y., Lobel, L., Hengartner, M., Horvitz, H. R. & Avery, L. Mutations in the α1 subunit of an L-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J. 16, 6066–6076 (1997)
Rogalski, T. M., Mullen, G. P., Gilbert, M. M., Williams, B. D. & Moerman, D. G. The unc-112 gene in Caenorhabditis elegans encodes a novel component of cell-matrix adhesion structures required for integrin localization in the muscle cell membrane. J. Cell Biol. 150, 253–264 (2000)
Bourne, H. R., Sanders, D. A. & McCormick, F. The GTPase superfamily: conserved structure and molecular mechanism. Nature 349, 117–127 (1991)
Gally, C. et al. Myosin II regulation during C. elegans embryonic elongation: LET-502/ROCK, MRCK-1 and PAK-1, three kinases with different roles. Development 136, 3109–3119 (2009)
Lecuit, T. & Lenne, P. F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nature Rev. Mol. Cell Biol. 8, 633–644 (2007)
Zhao, Z. S., Manser, E., Loo, T. H. & Lim, L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol. Cell. Biol. 20, 6354–6363 (2000)
Zhang, H., Webb, D. J., Asmussen, H., Niu, S. & Horwitz, A. F. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J. Neurosci. 25, 3379–3388 (2005)
Lucanic, M. & Cheng, H. J. A RAC/CDC-42-independent GIT/PIX/PAK signaling pathway mediates cell migration in C. elegans. PLoS Genet. 4, e1000269 (2008)
Giannone, G. & Sheetz, M. P. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 16, 213–223 (2006)
del Rio, A. et al. Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641 (2009)
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974)
Hodgkin, J. Male phenotypes and mating efficiency in Caenorhabditis elegans. Genetics 103, 43–64 (1983)
Reddien, P. W. & Horvitz, H. R. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nature Cell Biol. 2, 131–136 (2000)
Kaminsky, R. et al. SUMO regulates the assembly and function of a cytoplasmic intermediate filament protein in C. elegans. Dev. Cell 17, 724–735 (2009)
Kamath, R. S. et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237 (2003)
Gilleard, J. S., Barry, J. D. & Johnstone, I. L. cis regulatory requirements for hypodermal cell-specific expression of the Caenorhabditis elegans cuticle collagen gene dpy-7. Mol. Cell. Biol. 17, 2301–2311 (1997)
Anderson, D. C., Gill, J. S., Cinalli, R. M. & Nance, J. Polarization of the C. elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science 320, 1771–1774 (2008)
Gilleard, J. S., Shafi, Y., Barry, J. D. & McGhee, J. D. ELT-3: a Caenorhabditis elegans GATA factor expressed in the embryonic epidermis during morphogenesis. Dev. Biol. 208, 265–280 (1999)
Chen, W., Chen, S., Yap, S. F. & Lim, L. The Caenorhabditis elegans p21-activated kinase (CePAK) colocalizes with CeRac1 and CDC42Ce at hypodermal cell boundaries during embryo elongation. J. Biol. Chem. 271, 26362–26368 (1996)
Francis, R. & Waterston, R. H. Muscle cell attachment in Caenorhabditis elegans. J. Cell Biol. 114, 465–479 (1991)
Schnabel, R. Duels without obvious sense: counteracting inductions involved in body wall muscle development in the Caenorhabditis elegans embryo. Development 121, 2219–2232 (1995)
Karabinos, A., Schmidt, H., Harborth, J., Schnabel, R. & Weber, K. Essential roles for four cytoplasmic intermediate filament proteins in Caenorhabditis elegans development. Proc. Natl Acad. Sci. USA 98, 7863–7868 (2001)
Karabinos, A., Schulze, E., Schunemann, J., Parry, D. A. & Weber, K. In vivo and in vitro evidence that the four essential intermediate filament (IF) proteins A1, A2, A3 and B1 of the nematode Caenorhabditis elegans form an obligate heteropolymeric IF system. J. Mol. Biol. 333, 307–319 (2003)
Hapiak, V. et al. mua-6, a gene required for tissue integrity in Caenorhabditis elegans, encodes a cytoplasmic intermediate filament. Dev. Biol. 263, 330–342 (2003)
Iannuccelli, E. et al. NEMO: a tool for analyzing gene and chromosome territory distributions from 3D-FISH experiments. Bioinformatics 26, 696–697 (2010)
Acknowledgements
We are grateful to H.-J. Cheng, the CGC and NBP-Japan, L. Lim, J. Nance, C. Gally and L. Broday for reagents, as well as M. Argentini for technical advice. We thank J. Ahringer for a discussion, and O. Pourquié, J.-L. Bessereau, S. Jarriault and members of the Labouesse laboratory (C. Gally, I. Kolotueva, N. Osmani and S. Quintin) for critical reading of the manuscript. This work was supported by grants from the ANR, ARC and EU (STREP-FP6 programme) (M.L.) and by institutional funds from the CNRS and INSERM.
Author information
Authors and Affiliations
Contributions
H. Zhang and M.L. designed the study, analysed the data and wrote the paper. H. Zhang conducted most of the experiments. F.L. and H. Zahreddine made some initial observations (tension-change modification of the epidermis, and PAK-1 distribution and mutant phenotype) that proved to be essential for designing the study. D.R. provided technical help. M.K. helped to design and analyse the pressing experiment.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Table 1 and Supplementary Figures 1-10 with legends. (PDF 5847 kb)
Supplementary Movie 1
This file contains Supplementary Movie 1 – see Supplementary Information file page 19 for full legend. (MOV 1183 kb)
Supplementary Movie 2
This file contains Supplementary Movie 2 – see Supplementary Information file page 19 for full legend. (MOV 997 kb)
Supplementary Movie 3
This file contains Supplementary Movie 3 – see Supplementary Information file page 19 for full legend. (MOV 494 kb)
Supplementary Movie 4
This file contains Supplementary Movie 4 – see Supplementary Information file page 19 for legend. (MOV 830 kb)
Supplementary Movie 5
This file contains Supplementary Movie 5 – see Supplementary Information file page 19 for full legend. (MOV 680 kb)
Supplementary Movie 6
This file contains Supplementary Movie 6 – see Supplementary Information file page 19 for full legend. (MOV 986 kb)
Supplementary Movie 7
This file contains Supplementary Movie 7 – see Supplementary Information file page 19 for full legend. (MOV 1189 kb)
Rights and permissions
About this article
Cite this article
Zhang, H., Landmann, F., Zahreddine, H. et al. A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature 471, 99–103 (2011). https://doi.org/10.1038/nature09765
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09765
This article is cited by
-
The long protostomic-type cytoplasmic intermediate filament (cIF) protein in Branchiostoma supports the phylogenetic transition between the protostomic- and the chordate-type cIFs
Protoplasma (2023)
-
The first embryo, the origin of cancer and animal phylogeny. II. The neoplastic process as an evolutionary engine
Journal of Biosciences (2023)
-
KIAA0319 influences cilia length, cell migration and mechanical cell–substrate interaction
Scientific Reports (2022)
-
Generic self-stabilization mechanism for biomolecular adhesions under load
Nature Communications (2022)
-
Cell stretching is amplified by active actin remodelling to deform and recruit proteins in mechanosensitive structures
Nature Cell Biology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.