Abstract
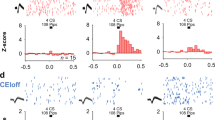
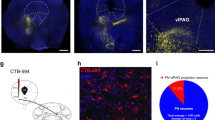
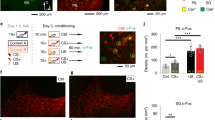
The central amygdala (CEA), a nucleus predominantly composed of GABAergic inhibitory neurons, is essential for fear conditioning. How the acquisition and expression of conditioned fear are encoded within CEA inhibitory circuits is not understood. Using in vivo electrophysiological, optogenetic and pharmacological approaches in mice, we show that neuronal activity in the lateral subdivision of the central amygdala (CEl) is required for fear acquisition, whereas conditioned fear responses are driven by output neurons in the medial subdivision (CEm). Functional circuit analysis revealed that inhibitory CEA microcircuits are highly organized and that cell-type-specific plasticity of phasic and tonic activity in the CEl to CEm pathway may gate fear expression and regulate fear generalization. Our results define the functional architecture of CEA microcircuits and their role in the acquisition and regulation of conditioned fear behaviour.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
LeDoux, J. E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000)
Davis, M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In The Amygdala (ed., Aggleton, J. P.) 213–288 (Oxford Univ. Press, 2000)
Maren, S. & Quirk, G. J. Neuronal signalling of fear memory. Nature Rev. Neurosci. 5, 844–852 (2004)
Sigurdsson, T., Doyère, V., Cain, C. K. & LeDoux, J. E. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology 52, 215–227 (2007)
Sah, P., Westbrook, R. F. & Lüthi, A. Fear conditioning and long-term potentiation: what really is the connection? Ann. NY Acad. Sci. 1129, 88–95 (2008)
Krettek, J. E. & Price, J. L. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J. Comp. Neurol. 178, 255–279 (1978)
Veening, J. G., Swanson, L. W. & Sawchenko, P. E. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 303, 337–357 (1984)
LeDoux, J. E., Iwata, J., Cicchetti, P. & Reis, D. J. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 8, 2517–2529 (1988)
Pascoe, J. P. & Kapp, B. S. Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behav. Brain Res. 16, 117–133 (1985)
Goosens, K. A. & Maren, S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav. Neurosci. 117, 738–750 (2003)
Wilensky, A. E., Schafe, G. E., Kristensen, M. P. & LeDoux, J. E. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J. Neurosci. 26, 12387–12396 (2006)
Samson, R. D., Duvarci, S. & Paré, D. Synaptic plasticity in the central nucleus of the amygdala. Rev. Neurosci. 16, 287–302 (2005)
Ehrlich, I. et al. Amygdala inhibitory circuits and the control of fear memory. Neuron 62, 757–771 (2009)
Sun, N., Yi, H. & Cassell, M. D. Evidence for a GABAergic interface between cortical afferents and brainstem projection neurons in the rat central extended amygdala. J. Comp. Neurol. 340, 43–64 (1994)
Cassell, M. D., Freedman, L. J. & Shi, C. The intrinsic organization of the central extended amygdala. Ann. NY Acad. Sci. 877, 217–241 (1999)
Veinante, P. & Freund-Mercier, M. J. Branching patterns of central amygdaloid nucleus afferents in the rat: Single axon reconstructions. Ann. NY Acad. Sci. 985, 552–553 (2003)
Huber, D., Veinante, P. & Stoop, R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308, 245–248 (2005)
Roberto, M., Madamba, S. G., Moore, S. D., Tallent, M. K. & Siggins, G. R. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc. Natl Acad. Sci. USA 100, 2053–2058 (2003)
Gradinaru, V. et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo . J. Neurosci. 27, 14231–14238 (2007)
Tang, W. et al. Faithful expression of multiple proteins via 2A-peptide self-processing: A versatile and reliable method for manipulating brain circuits. J. Neurosci. 29, 8621–8629 (2009)
Herry, C. et al. Switching on and off fear by distinct neuronal circuits. Nature 454, 600–606 (2008)
LeDoux, J. E., Ruggiero, D. A. & Reis, D. J. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J. Comp. Neurol. 242, 182–213 (1985)
Turner, B. H. & Herkenham, M. Thalamoamygdaloid projections in the rat: a test of the amygdala’s role in sensory processing. J. Comp. Neurol. 313, 295–325 (1991)
Linke, R., Braune, G. & Schwegler, H. Differential projection of the posterior paralaminar thalamic nuclei to the amygdaloid complex in the rat. Exp. Brain Res. 134, 520–532 (2000)
Lima, S. Q., Hromadka, T., Znamenskiy, P. & Zador, A. M. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS ONE 4, e6099 (2009)
Pitkänen, A., Savander, V. & LeDoux, J. E. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 20, 517–523 (1997)
Haubensak, W. et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature doi:10.1038/nature09553 (this issue).
Delaney, A. J., Crane, J. W. & Sah, P. Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism. Neuron 56, 880–892 (2007)
Fu, Y. & Shinnick-Gallagher, P. Two intra-amygdaloid pathways to the central amygdala exhibit different mechanisms of long-term potentiation. J. Neurophysiol. 93, 3012–3015 (2005)
Lopez de Armentia, M. & Sah, P. Bidirectional synaptic plasticity at nociceptive afferents in the rat central amygdala. J. Physiol. (Lond.) 581, 961–970 (2007)
Samson, R. D. & Paré, D. Activity-dependent synaptic plasticity in the central nucleus of the amygdala. J. Neurosci. 25, 1847–1855 (2005)
Millhouse, O. E. The intercalated cells of the amygdala. J. Comp. Neurol. 247, 246–271 (1986)
Paré, D. & Smith, Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience 57, 1077–1090 (1993)
Paré, D., Quirk, G. J. & LeDoux, J. E. New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 92, 1–9 (2004)
Thompson, R. F. The role of the cerebral cortex in stimulus generalization. J. Comp. Physiol. Psychol. 55, 279–287 (1962)
Jarrell, T. W., Gentile, C. G., Romanski, L. M., McCabe, P. M. & Schneidermann, N. Involvement of cortical and thalamic auditory regions in retention of differential bradycardia conditioning to acoustic conditioned stimuli in rabbits. Brain Res. 412, 285–294 (1987)
Shaban, H. et al. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nature Neurosci. 9, 1028–1035 (2006)
Cardinal, R. N., Parkinson, J. A., Hall, J. & Everitt, B. J. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 26, 321–352 (2002)
Balleine, B. W. & Killcross, S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 29, 272–279 (2006)
Neugebauer, V., Galhardo, V., Maione, S. & Mackey, S. C. Forebrain pain mechanisms. Brain Res. Brain Res. Rev. 60, 226–242 (2009)
Jolkkonen, E., Miettinen, R., Pikkarainen, M. & Pitkänen, A. Projections from the amygdaloid complex to the magnocellular cholinergic basal forebrain in rat. Neuroscience 111, 133–149 (2002)
Gozzi, A. et al. A neural switch for active and passive fear. Neuron 67, 656–666 (2010)
Wickens, J. R., Arbuthnott, G. W. & Shindou, T. Simulation of GABA function in the basal ganglia: computational models of GABAergic mechanisms in basal ganglia function. Prog. Brain Res. 160, 313–329 (2007)
Nicolelis, M. A. L. et al. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc. Natl Acad. Sci. USA 100, 11041–11046 (2003)
Herry, C. et al. Processing of temporal unpredictability in human and animal amygdala. J. Neurosci. 27, 5958–5966 (2007)
Fujisawa, S., Amarasingham, A., Harrison, M. T. & Buzsaki, G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nature Neurosci. 11, 823–833 (2008)
Lang, E. J. & Paré, D. Synaptic and synaptically activated intrinsic conductances underlie inhibitory potentials in cat lateral amygdaloid projection neurons in vivo . J. Neurophysiol. 77, 353–363 (1997)
Lima, S. Q., Hromadka, T., Znamenskiy, P. & Zador, A. M. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS ONE 4, e6099 (2009)
Acknowledgements
We thank all members of the Lüthi laboratory for discussions and critical comments on the manuscript. This work was supported by grants from the Austrian Science Fund (FWF), the Swiss National Science Foundation, the Schering Foundation, the European Commission (Eurospin Project, Contract HEALTH-F2-2009-241498), the Indo Swiss Joint Research Programme, the BMBF (grant 01GQ0420 to BCCN Freiburg), Neurex Interreg-IV, the Volkswagen Stiftung, the Novartis Institutes for Biomedical Research, and the Novartis Research Foundation.
Author information
Authors and Affiliations
Contributions
S.C. and C.H. initiated the project. S.C., C.H., F.G., S.B.E.W. and C.M. performed the experiments. S.C., C.H., F.G., S.B.E.W., I.V., M.B.S. and A.L. analysed the data. K.D. and R.S. provided constructs and advice. S.C., C.H., F.G., S.B.E.W., I.E. and J.J.L. contributed to the experimental design and interpretation. A.L. conceived the project, contributed to the experimental design and interpretation, analysed data and wrote the manuscript. S.C. and C.H. contributed equally. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Notes, an additional reference and Supplementary Figures 1-16 with legends. (PDF 8275 kb)
Rights and permissions
About this article
Cite this article
Ciocchi, S., Herry, C., Grenier, F. et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282 (2010). https://doi.org/10.1038/nature09559
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09559
This article is cited by
-
Social buffering in rats reduces fear by oxytocin triggering sustained changes in central amygdala neuronal activity
Nature Communications (2024)
-
Suppressing fear in the presence of a safety cue requires infralimbic cortical signaling to central amygdala
Neuropsychopharmacology (2024)
-
Microglia govern the extinction of acute stress-induced anxiety-like behaviors in male mice
Nature Communications (2024)
-
Sex-specific effects of psychedelic drug exposure on central amygdala reactivity and behavioral responding
Translational Psychiatry (2023)
-
Somatostatin neurons in the central amygdala mediate anxiety by disinhibition of the central sublenticular extended amygdala
Molecular Psychiatry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.