Abstract
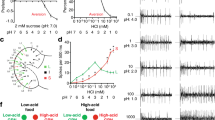
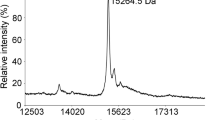
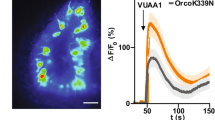
The odour of acids has a distinct quality that is perceived as sharp, pungent and often irritating1. How acidity is sensed and translated into an appropriate behavioural response is poorly understood. Here we describe a functionally segregated population of olfactory sensory neurons in the fruitfly, Drosophila melanogaster, that are highly selective for acidity. These olfactory sensory neurons express IR64a, a member of the recently identified ionotropic receptor (IR) family of putative olfactory receptors2. In vivo calcium imaging showed that IR64a+ neurons projecting to the DC4 glomerulus in the antennal lobe are specifically activated by acids. Flies in which the function of IR64a+ neurons or the IR64a gene is disrupted had defects in acid-evoked physiological and behavioural responses, but their responses to non-acidic odorants remained unaffected. Furthermore, artificial stimulation of IR64a+ neurons elicited avoidance responses. Taken together, these results identify cellular and molecular substrates for acid detection in the Drosophila olfactory system and support a labelled-line mode of acidity coding at the periphery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dravnieks, A. Odor quality: semantically generated multidimensional profiles are stable. Science 218, 799–801 (1982)
Benton, R., Vannice, K. S., Gomez-Diaz, C. & Vosshall, L. B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila . Cell 136, 149–162 (2009)
Ng, M. et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 36, 463–474 (2002)
Wang, J. W., Wong, A. M., Flores, J., Vosshall, L. B. & Axel, R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112, 271–282 (2003)
Kellogg, F. E. Water vapour and carbon dioxide receptors in Aedes aegypti . J. Insect Physiol. 16, 99–108 (1970)
Thom, C., Guerenstein, P. G., Mechaber, W. L. & Hildebrand, J. G. Floral CO2 reveals flower profitability to moths. J. Chem. Ecol. 30, 1285–1288 (2004)
Suh, G. S. et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila . Nature 431, 854–859 (2004)
Jones, W. D., Cayirlioglu, P., Kadow, I. G. & Vosshall, L. B. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila . Nature 445, 86–90 (2007)
Kwon, J. Y., Dahanukar, A., Weiss, L. A. & Carlson, J. R. The molecular basis of CO2 reception in Drosophila . Proc. Natl Acad. Sci. USA 104, 3574–3578 (2007)
Faucher, C., Forstreuter, M., Hilker, M. & de Bruyne, M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J. Exp. Biol. 209, 2739–2748 (2006)
Kitamoto, T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 47, 81–92 (2001)
Yorozu, S. et al. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature 458, 201–205 (2009)
Nakai, J., Ohkura, M. & Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nature Biotechnol. 19, 137–141 (2001)
Couto, A., Alenius, M. & Dickson, B. J. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 15, 1535–1547 (2005)
Shanbhag, S. R., Singh, K. & Singh, R. N. Fine structure and primary sensory projections of sensilla located in the sacculus of the antenna of Drosophila melanogaster . Cell Tissue Res. 282, 237–249 (1995)
Franz, G., Loukeris, T. G., Dialektaki, G., Thompson, C. R. & Savakis, C. Mobile Minos elements from Drosophila hydei encode a two-exon transposase with similarity to the paired DNA-binding domain. Proc. Natl Acad. Sci. USA 91, 4746–4750 (1994)
Sweeney, S. T., Broadie, K., Keane, J., Niemann, H. & O’Kane, C. J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14, 341–351 (1995)
Sambongi, Y. et al. Caenorhabditis elegans senses protons through amphid chemosensory neurons: proton signals elicit avoidance behavior. Neuroreport 11, 2229–2232 (2000)
Joseph, R. M., Devineni, A. V., King, I. F. & Heberlein, U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila . Proc. Natl Acad. Sci. USA 106, 11352–11357 (2009)
Ziemann, A. E. et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 139, 1012–1021 (2009)
Huang, A. L. et al. The cells and logic for mammalian sour taste detection. Nature 442, 934–938 (2006)
Vosshall, L. B., Wong, A. M. & Axel, R. An olfactory sensory map in the fly brain. Cell 102, 147–159 (2000)
Larsson, M. C. et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714 (2004)
Metaxakis, A., Oehler, S., Klinakis, A. & Savakis, C. Minos as a genetic and genomic tool in Drosophila melanogaster . Genetics 171, 571–581 (2005)
Asahina, K., Louis, M., Piccinotti, S. & Vosshall, L. B. A circuit supporting concentration-invariant odor perception in Drosophila . J. Biol. 8, 9 (2009)
Acknowledgements
We thank M. Dus for helpful discussions and technical support for making anti-IR64a antibody; M. Kim for technical assistance; J. Treisman, N. Ringstad, C. Desplan, M. Warman, G. Fishell, M. Chesler, A. Wong, L. Vosshall and D. Anderson for discussions and comments on the manuscript; and R. Lehmann for sharing her two-photon microscope. This work was initiated in D. Anderson’s laboratory at California Institute of Technology. G.S.B.S. thanks H.-R. Song for support. Financial support was provided by a National Research Service Award fellowship (M.A.), the Boehringer Ingelheim Foundation (R. Bell), the Centre National de la Recherche Scientifique (Y.G.), a European Research Council Starting Independent Researcher Grant (R. Benton), the Swiss National Science Foundation (R. Benton), the Alfred P. Sloan Foundation (G.S.), the Whitehall Foundation (G.S.), a Whitehead President Award (G.S.) and NIH grant 1RO1GM089746 (G.S).
Author information
Authors and Affiliations
Contributions
G.S.B.S. identified the GC16-GAL4 line from a screen and performed initial imaging experiments. M.A. generated most of the transgenic flies, performed calcium imaging and some behavioural experiments, and conducted immunohistochemistry. S.M. performed most of the behavioural testing and immunohistochemistry. C.L. assisted in characterizing IR64a mutant alleles. Y.G., R. Bell and R. Benton generated IR64a-GAL4 and UAS-IR64a transgenic flies and showed that IR64a+ neurons innervate the DC4 and DP1m glomeruli. M.A. and G.S.B.S. analysed and interpreted the data and wrote the manuscript with inputs from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-6 with legends and Supplementary Tables 1-2. (PDF 3927 kb)
Rights and permissions
About this article
Cite this article
Ai, M., Min, S., Grosjean, Y. et al. Acid sensing by the Drosophila olfactory system. Nature 468, 691–695 (2010). https://doi.org/10.1038/nature09537
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09537
This article is cited by
-
Odor-regulated oviposition behavior in an ecological specialist
Nature Communications (2023)
-
Genetic atlas of hygro-and thermosensory cells in the vinegar fly Drosophila melanogaster
Scientific Reports (2023)
-
Aggregation pheromones have a non-linear effect on oviposition behavior in Drosophila melanogaster
Nature Communications (2023)
-
Sensorimotor transformation underlying odor-modulated locomotion in walking Drosophila
Nature Communications (2023)
-
The power of Drosophila genetics in studying insect toxicology and chemical ecology
Crop Health (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.