Abstract
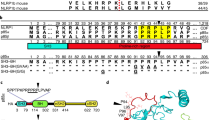
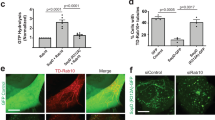
Bacillus anthracis is the causative agent of anthrax in humans and other mammals1,2. In lethal systemic anthrax, proliferating bacilli secrete large quantities of the toxins lethal factor (LF) and oedema factor (EF), leading to widespread vascular leakage and shock. Whereas host targets of LF (mitogen-activated protein-kinase kinases) and EF (cAMP-dependent processes)3 have been implicated in the initial phase of anthrax1,2, less is understood about toxin action during the final stage of infection. Here we use Drosophila melanogaster to identify the Rab11/Sec15 exocyst, which acts at the last step of endocytic recycling, as a novel target of both EF and LF. EF reduces levels of apically localized Rab11 and indirectly blocks vesicle formation by its binding partner and effector Sec15 (Sec15–GFP), whereas LF acts more directly to reduce Sec15–GFP vesicles. Convergent effects of EF and LF on Rab11/Sec15 inhibit expression of and signalling by the Notch ligand Delta and reduce DE-cadherin levels at adherens junctions. In human endothelial cells, the two toxins act in a conserved fashion to block formation of Sec15 vesicles, inhibit Notch signalling, and reduce cadherin expression at adherens junctions. This coordinated disruption of the Rab11/Sec15 exocyst by anthrax toxins may contribute to toxin-dependent barrier disruption and vascular dysfunction during B. anthracis infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mourez, M. Anthrax toxins. Rev. Physiol. Biochem. Pharmacol. 152, 135–164 (2004)
Tournier, J. N., Quesnel-Hellmann, A., Cleret, A. & Vidal, D. R. Contribution of toxins to the pathogenesis of inhalational anthrax. Cell. Microbiol. 9, 555–565 (2007)
Lacy, D. B. & Collier, R. J. Structure and function of anthrax toxin. Curr. Top. Microbiol. Immunol. 271, 61–85 (2002)
Duesbery, N. S. et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280, 734–737 (1998)
Vitale, G. et al. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem. Biophys. Res. Commun. 248, 706–711 (1998)
Leppla, S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl Acad. Sci. USA 79, 3162–3166 (1982)
Moayeri, M. & Leppla, S. H. The roles of anthrax toxin in pathogenesis. Curr. Opin. Microbiol. 7, 19–24 (2004)
Guichard, A., Park, J. M., Cruz-Moreno, B., Karin, M. & Bier, E. Anthrax lethal factor and edema factor act on conserved targets in Drosophila . Proc. Natl Acad. Sci. USA 103, 3244–3249 (2006)
Pezard, C., Berche, P. & Mock, M. Contribution of individual toxin components to virulence of Bacillus anthracis . Infect. Immun. 59, 3472–3477 (1991)
Fortini, M. E. & Bilder, D. Endocytic regulation of Notch signaling. Curr. Opin. Genet. Dev. 19, 323–328 (2009)
Jafar-Nejad, H. et al. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev. Cell 9, 351–363 (2005)
Wu, H., Rossi, G. & Brennwald, P. The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 18, 397–404 (2008)
Zhang, J. et al. Thirty-one flavors of Drosophila rab proteins. Genetics 176, 1307–1322 (2007)
Emery, G. et al. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell 122, 763–773 (2005)
Langevin, J. et al. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev. Cell 9, 365–376 (2005)
Guo, W., Roth, D., Walch-Solimena, C. & Novick, P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18, 1071–1080 (1999)
Salminen, A. & Novick, P. J. The Sec15 protein responds to the function of the GTP binding protein, Sec4, to control vesicular traffic in yeast. J. Cell Biol. 109, 1023–1036 (1989)
Zhang, X. M., Ellis, S., Sriratana, A., Mitchell, C. A. & Rowe, T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J. Biol. Chem. 279, 43027–43034 (2004)
Wu, S., Mehta, S. Q., Pichaud, F., Bellen, H. J. & Quiocho, F. A. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo . Nature Struct. Mol. Biol. 12, 879–885 (2005)
Roca, C. & Adams, R. H. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 21, 2511–2524 (2007)
Dejana, E., Tournier-Lasserve, E. & Weinstein, B. M. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev. Cell 16, 209–221 (2009)
Janes, B. K. & Stibitz, S. Routine markerless gene replacement in Bacillus anthracis . Infect. Immun. 74, 1949–1953 (2006)
Firoved, A. M. et al. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am. J. Pathol. 167, 1309–1320 (2005)
Kuo, S. R. et al. Anthrax toxin-induced shock in rats is associated with pulmonary edema and hemorrhage. Microb. Pathog. 44, 467–472 (2007)
Gozes, Y., Moayeri, M., Wiggins, J. F. & Leppla, S. H. Anthrax lethal toxin induces ketotifen-sensitive intradermal vascular leakage in certain inbred mice. Infect. Immun. 74, 1266–1272 (2006)
Tessier, J. et al. Contributions of histamine, prostanoids, and neurokinins to edema elicited by edema toxin from Bacillus anthracis . Infect. Immun. 75, 1895–1903 (2007)
Balzac, F. et al. E-cadherin endocytosis regulates the activity of Rap1: a traffic light GTPase at the crossroads between cadherin and integrin function. J. Cell Sci. 118, 4765–4783 (2005)
Silvis, M. R. et al. Rab11b regulates the apical recycling of the cystic fibrosis transmembrane conductance regulator in polarized intestinal epithelial cells. Mol. Biol. Cell 20, 2337–2350 (2009)
Kosman, D. et al. Multiplex detection of RNA expression in Drosophila embryos. Science 305, 846 (2004)
van Sorge, N. M. et al. Anthrax toxins inhibit neutrophil signaling pathways in brain endothelium and contribute to the pathogenesis of meningitis. PLoS ONE 3, e2964 (2008)
Cook, O., Biehs, B. & Bier, E. brinker and optomotor-blind act coordinately to initiate development of the L5 wing vein primordium in Drosophila . Development 131, 2113–2124 (2004)
Lunde, K. et al. Activation of the knirps locus links patterning to morphogenesis of the second wing vein in Drosophila . Development 130, 235–248 (2003)
O’Neill, J. W. & Bier, E. Double-label in situ hybridization using biotin and digoxigenin-tagged RNA probes. Biotechniques 17, 874–875 (1994)
Acknowledgements
We thank A. Kurciyan for help in analysing the effects of expressing wild-type and dominant-negative forms of Rab in Drosophila, A. Cooper and members of the E.B. and V.N. laboratories and H. Bellen for comments on the manuscript and suggestions. We thank S. Leppla for providing purified preparations of LF, EF and PA, S. Stibitz for B. anthracis mutants, and the following investigators for providing antibodies: R. Cohen (anti-Rab11), A. Parks (anti-Dl), K. Irvine (anti-Serrate) and J. Collier (anti-LF). Support for these studies was provided by National Institutes of Health (NIH) R01 grants AI070654 and NS29870 (E.B.), AI077780 (V.N.), an IRACDA NIH postdoctoral fellowship GM068524 (S.M.M.) and a Biomedical Research Fellowship from The Hartwell Foundation (S.M.M.).
Author information
Authors and Affiliations
Contributions
All authors participated in designing the experiments. A.G. and B.C.-M. carried out the Drosophila experiments. S.M.M. and N.M.v.S. carried out the experiments with vertebrate cells and mice. E.B. wrote the manuscript with all other authors providing significant input.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-12 with legends, Supplementary Tables 1-2, Supplementary Results, an additional reference and a list of Supplementary Microscopy Settings. (PDF 23277 kb)
Rights and permissions
About this article
Cite this article
Guichard, A., McGillivray, S., Cruz-Moreno, B. et al. Anthrax toxins cooperatively inhibit endocytic recycling by the Rab11/Sec15 exocyst. Nature 467, 854–858 (2010). https://doi.org/10.1038/nature09446
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09446
This article is cited by
-
Autocrine–paracrine prostaglandin E2 signaling restricts TLR4 internalization and TRIF signaling
Nature Immunology (2018)
-
Anthrax immune globulin improves hemodynamics and survival during B. anthracis toxin-induced shock in canines receiving titrated fluid and vasopressor support
Intensive Care Medicine Experimental (2017)
-
Raxibacumab augments hemodynamic support and improves outcomes during shock with B. anthracis edema toxin alone or together with lethal toxin in canines
Intensive Care Medicine Experimental (2015)
-
RAB11-mediated trafficking in host–pathogen interactions
Nature Reviews Microbiology (2014)
-
Phenotypic and functional consequences of haploinsufficiency of genes from exocyst and retinoic acid pathway due to a recurrent microdeletion of 2p13.2
Orphanet Journal of Rare Diseases (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.