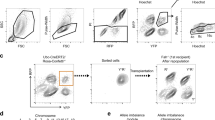
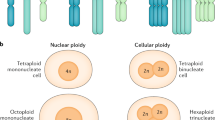
Abstract
Mononucleated and binucleated polyploid hepatocytes (4n, 8n, 16n and higher) are found in all mammalian species, but the functional significance of this conserved phenomenon remains unknown1,2,3,4. Polyploidization occurs through failed cytokinesis, begins at weaning in rodents and increases with age2,5,6,7. Previously, we demonstrated that the opposite event, ploidy reversal, also occurs in polyploid hepatocytes generated by artificial cell fusion8,9,10. This raised the possibility that somatic ‘reductive mitoses’ can also happen in normal hepatocytes. Here we show that multipolar mitotic spindles form frequently in mouse polyploid hepatocytes and can result in one-step ploidy reversal to generate offspring with halved chromosome content. Proliferating hepatocytes produce a highly diverse population of daughter cells with multiple numerical chromosome imbalances as well as uniparental origins. Our findings support a dynamic model of hepatocyte polyploidization, ploidy reversal and aneuploidy, a phenomenon that we term the ‘ploidy conveyor’. We propose that this mechanism evolved to generate genetic diversity and permits adaptation of hepatocytes to xenobiotic or nutritional injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Faktor, V. M. & Uryvaeva, I. V. Progressive polyploidy in mouse liver following repeated hepatectomy. Tsitologiia 17, 909–916 (1975)
Guidotti, J. E. et al. Liver cell polyploidization: a pivotal role for binuclear hepatocytes. J. Biol. Chem. 278, 19095–19101 (2003)
Kudryavtsev, B. N., Kudryavtseva, M. V., Sakuta, G. A. & Stein, G. I. Human hepatocyte polyploidization kinetics in the course of life cycle. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 64, 387–393 (1993)
Yim, A. P. Some flow-cytofluorimetric studies of the nuclear ploidy of mouse hepatocytes: iii. further observations on early changes in nuclear ploidy of mouse hepatocytes following various experimental procedures. Br. J. Exp. Pathol. 63, 458–461 (1982)
Barbason, H., Van Cantfort, J. & Houbrechts, N. Correlation between tissular and division functions in the liver of young rats. Cell Tissue Kinet. 7, 319–326 (1974)
Celton-Morizur, S., Merlen, G., Couton, D., Margall-Ducos, G. & Desdouets, C. The insulin/Akt pathway controls a specific cell division program that leads to generation of binucleated tetraploid liver cells in rodents. J. Clin. Invest. 119, 1880–1887 (2009)
Margall-Ducos, G., Celton-Morizur, S., Couton, D., Bregerie, O. & Desdouets, C. Liver tetraploidization is controlled by a new process of incomplete cytokinesis. J. Cell Sci. 120, 3633–3639 (2007)
Duncan, A. W. et al. Ploidy reductions in murine fusion-derived hepatocytes. PLoS Genet. 5, e1000385 (2009)
Wang, X. et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 422, 897–901 (2003)
Willenbring, H. et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nature Med. 10, 744–748 (2004)
Overturf, K. et al. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nature Genet. 12, 266–273 (1996)
Jorquera, R. & Tanguay, R. M. The mutagenicity of the tyrosine metabolite, fumarylacetoacetate, is enhanced by glutathione depletion. Biochem. Biophys. Res. Commun. 232, 42–48 (1997)
Yannoutsos, N. et al. A membrane cofactor protein transgenic mouse model for the study of discordant xenograft rejection. Genes Cells 1, 409–419 (1996)
Ganem, N. J., Godinho, S. A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 (2009)
Gimelbrant, A., Hutchinson, J. N., Thompson, B. R. & Chess, A. Widespread monoallelic expression on human autosomes. Science 318, 1136–1140 (2007)
Rancati, G. et al. Aneuploidy underlies rapid adaptive evolution of yeast cells deprived of a conserved cytokinesis motor. Cell 135, 879–893 (2008)
Manning, K., Al-Dhalimy, M., Finegold, M. & Grompe, M. In vivo suppressor mutations correct a murine model of hereditary tyrosinemia type I. Proc. Natl Acad. Sci. USA 96, 11928–11933 (1999)
Mitchell, C. & Willenbring, H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nature Protocols 3, 1167–1170 (2008)
Ko, M. A. et al. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nature Genet. 37, 883–888 (2005)
Darlington, G. J., Kelley, J. H. & Buffone, G. J. Growth and hepatospecific gene expression of human hepatoma cells in a defined medium. In Vitro Cell. Dev. Biol. 23, 349–354 (1987)
Bayani, J. & Squire, J. A. Fluorescence in situ hybridization (FISH). Curr. Protoc. Cell Biol. 10.1002/0471143030.cb2204s23 22 (2004)
Overturf, K. et al. Adenovirus-mediated gene therapy in a mouse model of hereditary tyrosinemia type I. Hum. Gene Ther. 8, 513–521 (1997)
Pagano, M., Pepperkok, R., Verde, F., Ansorge, W. & Draetta, G. Cyclin A is required at two points in the human cell cycle. EMBO J. 11, 961–971 (1992)
Friedrich, G. & Soriano, P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5, 1513–1523 (1991)
Grompe, M. et al. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev. 7, 2298–2307 (1993)
Lagasse, E. et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo . Nature Med. 6, 1229–1234 (2000)
Acknowledgements
We thank P. Canaday (Flow Cytometry Resource at OHSU) for cell sorting; A. Snyder and S. Kaech Petrie (Advanced Light Microscopy Core at OHSU, Core grant S10-RR023432) for microscopy assistance; and the Morphology Core of the Texas Medical Center (DK56338) for histology support. We also thank L. Smith and M. Thayer for discussions. This work was supported by grants from the National Institute of Health to M.G. (R01DK067636) and A.W.D. (F32DK076232).
Author information
Authors and Affiliations
Contributions
A.W.D. designed and performed most of the experiments, analysed data and wrote the paper. M.H.T. helped with imaging of dividing hepatocytes. R.D.H. assisted with data analysis. A.E.H.N., M.L.L. and S.B.O. performed all of the cytogenetic analyses. Histological analyses were performed by M.J.F. M.G. supervised all aspects of this work. All authors discussed the results and edited the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-14 with legends. (PDF 6377 kb)
Supplementary Movie 1
This movie shows a single binucleated tetraploid hepatocyte undergoing bipolar mitosis. Successful cytokinesis produces 2 mononucleated daughter cells. Time-lapse sequence is annotated in Supplementary Figure 9. (MOV 3051 kb)
Supplementary Movie 2
This movie shows a single mononucleated tetraploid hepatocyte undergoing bipolar mitosis. Failed cytokinesis produces a single binucleated cell. Time-lapse sequence is annotated in Supplementary Figure 10. (MOV 1835 kb)
Supplementary Movie 3
This movie shows a single binucleated tetraploid hepatocyte undergoing tripolar division. Partial failed cytokinesis generates a mononucleated daughter and a binucleated daughter. Time-lapse sequence is annotated in Figure 4b. (MOV 1739 kb)
Supplementary Movie 4
This movie shows a single binucleated tetraploid hepatocyte undergoing tripolar division. Partial failed cytokinesis generates a mononucleated daughter and a binucleated daughter. Both daughter cells proceed to divide again, producing 2 mononucleated daughters each. Time-lapse sequence is annotated in Supplementary Figure 11. (MOV 5689 kb)
Supplementary Movie 5
This movie shows a binucleated tetraploid hepatocyte undergoing double mitosis. Adjacent nuclei migrate apart prior to entering mitosis. Each nucleus undergoes a distinct mitosis, generating 4 daughter nuclei. Successful cytokinesis produces 4 mononucleated daughter cells. Time-lapse sequence is annotated in Supplementary Figure 13. (MOV 2910 kb)
Supplementary Movie 6
This movie shows a mononucleated tetraploid hepatocyte undergoing double mitosis. Following nuclear breakdown, chromosomes align along 2 discrete metaphase plates, which undergo simultaneous bipolar anaphase to produce 4 daughter nuclei. Cytokinesis is partially successful, generating 2 mononucleated daughters and 1 binucleated daughter. Time-lapse sequence is annotated in Supplementary Figure 14. (MOV 2171 kb)
Rights and permissions
About this article
Cite this article
Duncan, A., Taylor, M., Hickey, R. et al. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature 467, 707–710 (2010). https://doi.org/10.1038/nature09414
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09414
This article is cited by
-
Intrinsic signalling factors associated with cancer cell-cell fusion
Cell Communication and Signaling (2023)
-
RIT1 regulates mitosis and promotes proliferation by interacting with SMC3 and PDS5 in hepatocellular carcinoma
Journal of Experimental & Clinical Cancer Research (2023)
-
Targeting anillin inhibits tumorigenesis and tumor growth in hepatocellular carcinoma via impairing cytokinesis fidelity
Oncogene (2022)
-
IL6 supports long-term expansion of hepatocytes in vitro
Nature Communications (2022)
-
Single-cell resolved ploidy and chromosomal aberrations in nonalcoholic steatohepatitis-(NASH) induced hepatocellular carcinoma and its precursor lesions
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.