Abstract
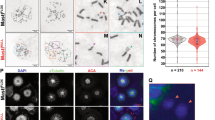
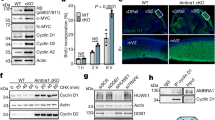
Cell cycle checkpoints are implemented to safeguard the genome, avoiding the accumulation of genetic errors1,2. Checkpoint loss results in genomic instability and contributes to the evolution of cancer. Among G1-, S-, G2- and M-phase checkpoints, genetic studies indicate the role of an intact S-phase checkpoint in maintaining genome integrity3,4. Although the basic framework of the S-phase checkpoint in multicellular organisms has been outlined, the mechanistic details remain to be elucidated. Human chromosome-11 band-q23 translocations disrupting the MLL gene lead to poor prognostic leukaemias5,6,7,8,9. Here we assign MLL as a novel effector in the mammalian S-phase checkpoint network and identify checkpoint dysfunction as an underlying mechanism of MLL leukaemias. MLL is phosphorylated at serine 516 by ATR in response to genotoxic stress in the S phase, which disrupts its interaction with, and hence its degradation by, the SCFSkp2 E3 ligase, leading to its accumulation. Stabilized MLL protein accumulates on chromatin, methylates histone H3 lysine 4 at late replication origins and inhibits the loading of CDC45 to delay DNA replication. Cells deficient in MLL showed radioresistant DNA synthesis and chromatid-type genomic abnormalities, indicative of S-phase checkpoint dysfunction. Reconstitution of Mll−/− (Mll also known as Mll1) mouse embryonic fibroblasts with wild-type but not S516A or ΔSET mutant MLL rescues the S-phase checkpoint defects. Moreover, murine myeloid progenitor cells carrying an Mll–CBP knock-in allele that mimics human t(11;16) leukaemia show a severe radioresistant DNA synthesis phenotype. MLL fusions function as dominant negative mutants that abrogate the ATR-mediated phosphorylation/stabilization of wild-type MLL on damage to DNA, and thus compromise the S-phase checkpoint. Together, our results identify MLL as a key constituent of the mammalian DNA damage response pathway and show that deregulation of the S-phase checkpoint incurred by MLL translocations probably contributes to the pathogenesis of human MLL leukaemias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zhou, B.-B. S. & Elledge, S. J. The DNA damage response: putting checkpoints in perspective. Nature 408, 433–439 (2000)
Kastan, M. B. & Bartek, J. Cell-cycle checkpoints and cancer. Nature 432, 316–323 (2004)
Bartek, J., Lukas, C. & Lukas, J. Checking on DNA damage in S phase. Nature Rev. Mol. Cell Biol. 5, 792–804 (2004)
Kolodner, R. D., Putnam, C. D. & Myung, K. Maintenance of genome stability in Saccharomyces cerevisiae. Science 297, 552–557 (2002)
Krivtsov, A. V. & Armstrong, S. A. MLL translocations, histone modifications and leukaemia stem-cell development. Natl. Rev. 7, 823–833 (2007)
Rodriguez-Perales, S., Cano, F., Lobato, M. N. & Rabbitts, T. H. MLL gene fusions in human leukaemias: in vivo modelling to recapitulate these primary tumourigenic events. Int. J. Hematol. 87, 3–9 (2008)
Liu, H., Cheng, E. H. & Hsieh, J. J. MLL fusions: pathways to leukemia. Cancer Biol. Ther. 8, 1204–1211 (2009)
Meyer, C. et al. New insights to the MLL recombinome of acute leukemias. Leukemia 23, 1490–1499 (2009)
Liedtke, M. & Cleary, M. L. Therapeutic targeting of MLL. Blood 113, 6061–6068 (2009)
Hsieh, J. J., Cheng, E. H. & Korsmeyer, S. J. Taspase1: a threonine aspartase required for cleavage of MLL and proper HOX gene expression. Cell 115, 293–303 (2003)
Jude, C. D. et al. Unique and independent roles for MLL in adult hematopoietic stem cells and progenitors. Cell Stem Cell 1, 324–337 (2007)
Liu, H., Cheng, E. H. & Hsieh, J. J. Bimodal degradation of MLL by SCFSkp2 and APCCdc20 assures cell cycle execution: a critical regulatory circuit lost in leukemogenic MLL fusions. Genes Dev. 21, 2385–2398 (2007)
Takeda, S. et al. Proteolysis of MLL family proteins is essential for taspase1-orchestrated cell cycle progression. Genes Dev. 20, 2397–2409 (2006)
Yu, B. D., Hess, J. L., Horning, S. E., Brown, G. A. & Korsmeyer, S. J. Altered Hox expression and segmental identity in Mll-mutant mice. Nature 378, 505–508 (1995)
Milne, T. A. et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol. Cell 10, 1107–1117 (2002)
Kumar, A. R. et al. Hoxa9 influences the phenotype but not the incidence of Mll-AF9 fusion gene leukemia. Blood 103, 1823–1828 (2004)
Milne, T. A. et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc. Natl Acad. Sci. USA 102, 749–754 (2005)
Xia, Z. B. et al. The MLL fusion gene, MLL-AF4, regulates cyclin-dependent kinase inhibitor CDKN1B (p27kip1) expression. Proc. Natl Acad. Sci. USA 102, 14028–14033 (2005)
Tyagi, S., Chabes, A. L., Wysocka, J. & Herr, W. E2F activation of S phase promoters via association with HCF-1 and the MLL family of histone H3K4 methyltransferases. Mol. Cell 27, 107–119 (2007)
Zinkel, S. S. et al. A role for proapoptotic BID in the DNA-damage response. Cell 122, 579–591 (2005)
Wang, J. et al. Conditional MLL-CBP targets GMP and models therapy-related myeloproliferative disease. EMBO J. 24, 368–381 (2005)
Eguchi, M. et al. MLL chimeric protein activation renders cells vulnerable to chromosomal damage: an explanation for the very short latency of infant leukemia. Genes Chromosom. Cancer 45, 754–760 (2006)
Brown, E. J. & Baltimore, D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 17, 615–628 (2003)
Taccioli, G. E. et al. Targeted disruption of the catalytic subunit of the DNA-PK gene in mice confers severe combined immunodeficiency and radiosensitivity. Immunity 9, 355–366 (1998)
Cimprich, K. A. & Cortez, D. ATR: an essential regulator of genome integrity. Nature Rev. Mol. Cell Biol. 9, 616–627 (2008)
Arias, E. E. & Walter, J. C. Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells. Genes Dev. 21, 497–518 (2007)
Santocanale, C. & Diffley, J. F. X. Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 395, 615–618 (1998)
Sheu, Y. J. & Stillman, B. Cdc7-Dbf4 phosphorylates MCM proteins via a docking site-mediated mechanism to promote S phase progression. Mol. Cell 24, 101–113 (2006)
Goren, A., Tabib, A., Hecht, M. & Cedar, H. DNA replication timing of the human beta-globin domain is controlled by histone modification at the origin. Genes Dev. 22, 1319–1324 (2008)
Lim, D.-S. et al. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature 404, 613–617 (2000)
Liu, K., Paik, J. C., Wang, B., Lin, F. T. & Lin, W. C. Regulation of TopBP1 oligomerization by Akt/PKB for cell survival. EMBO J. 25, 4795–4807 (2006)
Hsieh, J. J., Ernst, P., Erdjument-Bromage, H., Tempst, P. & Korsmeyer, S. J. Proteolytic cleavage of MLL generates a complex of N- and C-terminal fragments that confers protein stability and subnuclear localization. Mol. Cell. Biol. 23, 186–194 (2003)
Kim, H. et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nature Cell Biol. 8, 1348–1358 (2006)
Yu, B. D., Hanson, R. D., Hess, J. L., Horning, S. E. & Korsmeyer, S. J. MLL, a mammalian trithorax-group gene, functions as a transcriptional maintenance factor in morphogenesis. Proc. Natl Acad. Sci. USA 95, 10632–10636 (1998)
Theunissen, J. W. & Petrini, J. H. Methods for studying the cellular response to DNA damage: influence of the Mre11 complex on chromosome metabolism. Methods Enzymol. 409, 251–284 (2006)
Tu, H. C. et al. The p53-cathepsin axis cooperates with ROS to activate programmed necrotic death upon DNA damage. Proc. Natl Acad. Sci. USA 106, 1093–1098 (2009)
Gupta, A. et al. The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Mol. Cell. Biol. 28, 397–409 (2008)
Méndez, J. & Stillman, B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 20, 8602–8612 (2000)
Acknowledgements
We thank J. Y. Wang and Z. You for discussions during the inception and the completion of this study, respectively. H.L. is supported by the Scholar award of the American Society of Hematology. The Mll+/ex7(stop)CBP mice were provided by S. Armstrong and the late S. Korsmeyer. This study is supported by CA119008, the Scholar award of the American Society of Hematology, the Scholar award of the American Cancer Society, to J.J.-D.H., and CA129537/CA123232, to T.K.P.
Author information
Authors and Affiliations
Contributions
H.L. designed and performed the experiments; T.K.P. designed some experiments; S.T., R.K. and T.D.W. performed some experiments; E.J.B. generated essential tools; and E.H.-Y.C. and J.J.-D.H. designed the experiments and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion, Supplementary Figures 1-13 with legends and additional references. (PDF 1772 kb)
Rights and permissions
About this article
Cite this article
Liu, H., Takeda, S., Kumar, R. et al. Phosphorylation of MLL by ATR is required for execution of mammalian S-phase checkpoint. Nature 467, 343–346 (2010). https://doi.org/10.1038/nature09350
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09350
This article is cited by
-
Protein phosphatase 1 regulatory subunit 15 A promotes translation initiation and induces G2M phase arrest during cuproptosis in cancers
Cell Death & Disease (2024)
-
The phosphorylation to acetylation/methylation cascade in transcriptional regulation: how kinases regulate transcriptional activities of DNA/histone-modifying enzymes
Cell & Bioscience (2022)
-
Targeting matrix metallopeptidase 2 by hydroxyurea selectively kills acute myeloid mixed-lineage leukemia
Cell Death Discovery (2022)
-
Bromodomain proteins: protectors against endogenous DNA damage and facilitators of genome integrity
Experimental & Molecular Medicine (2021)
-
Restoration of microRNA function impairs MYC-dependent maintenance of MLL leukemia
Leukemia (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.