Abstract
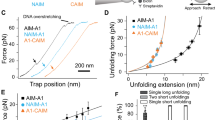
Haemostasis in the arteriolar circulation mediated by von Willebrand factor (VWF) binding to platelets is an example of an adhesive interaction that must withstand strong hydrodynamic forces acting on cells. VWF is a concatenated, multifunctional protein that has binding sites for platelets as well as subendothelial collagen1,2. Binding of the A1 domain in VWF to the glycoprotein Ib α subunit (GPIbα) on the surface of platelets mediates crosslinking of platelets to one another and the formation of a platelet plug for arterioles3,4. The importance of VWF is illustrated by its mutation in von Willebrand disease, a bleeding diathesis1. Here, we describe a novel mechanochemical specialization of the A1–GPIbα bond for force-resistance. We have developed a method that enables, for the first time, repeated measurements of the binding and unbinding of a receptor and ligand in a single molecule (ReaLiSM). We demonstrate two states of the receptor–ligand bond, that is, a flex-bond. One state is seen at low force; a second state begins to engage at 10 pN with a ∼20-fold longer lifetime and greater force resistance. The lifetimes of the two states, how force exponentiates lifetime, and the kinetics of switching between the two states are all measured. For the first time, single-molecule measurements on this system are in agreement with bulk phase measurements. The results have important implications not only for how platelets bound to VWF are able to resist force to plug arterioles, but also how increased flow activates platelet plug formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sadler, J. E. New concepts in von Willebrand disease. Annu. Rev. Med. 56, 173–191 (2005)
Ruggeri, Z. M. & Mendolicchio, G. L. Adhesion mechanisms in platelet function. Circ. Res. 100, 1673–1685 (2007)
Ruggeri, Z. M., Orje, J. N., Habermann, R., Federici, A. B. & Reininger, A. J. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood 108, 1903–1910 (2006)
Nesbitt, W. S. et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nature Med. 15, 665–673 (2009)
Huizinga, E. G. et al. Structures of glycoprotein Ibα and its complex with von Willebrand factor A1 domain. Science 297, 1176–1179 (2002)
Dumas, J. J. et al. Crystal structure of the wild-type von Willebrand factor A1-glycoprotein Ibα complex reveals conformation differences with a complex bearing von Willebrand disease mutations. J. Biol. Chem. 279, 23327–23334 (2004)
Bustamante, C. & Smith, S. Light-force sensor and method for measuring axial optical-trap forces from changes in light momentum along an optic axis. US Patent 7,133. 132 (2004)
Bustamante, C., Smith, S. B., Liphardt, J. & Smith, D. Single-molecule studies of DNA mechanics. Curr. Opin. Struct. Biol. 10, 279–285 (2000)
Evans, E. & Ritchie, K. Strength of a weak bond connecting flexible polymer chains. Biophys. J. 76, 2439–2447 (1999)
Dudko, O. K., Hummer, G. & Szabo, A. Theory, analysis, and interpretation of single-molecule force spectroscopy experiments. Proc. Natl Acad. Sci. USA 105, 15755–15760 (2008)
Bell, G. I. Models for the specific adhesion of cells to cells: a theoretical framework for adhesion mediated by reversible bonds between cell surface molecules. Science 200, 618–627 (1978)
Miura, S. et al. Interaction of von Willebrand factor domain A1 with platelet glycoprotein Ibα-(1–289). Slow intrinsic binding kinetics mediate rapid platelet adhesion. J. Biol. Chem. 275, 7539–7546 (2000)
Dong, J. F. et al. Ristocetin-dependent, but not botrocetin-dependent, binding of von Willebrand factor to the platelet glycoprotein Ib-IX-V complex correlates with shear-dependent interactions. Blood 97, 162–168 (2001)
De Luca, M. et al. Structure and function of the von Willebrand factor A1 domain: analysis with monoclonal antibodies reveals distinct binding sites involved in recognition of the platelet membrane glycoprotein Ib-IX-V complex and ristocetin-dependent activation. Blood 95, 164–172 (2000)
Dembo, M., Torney, D. C., Saxman, K. & Hammer, D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc. R. Soc. Lond. B 234, 55–83 (1988)
Phan, U. T., Waldron, T. T. & Springer, T. A. Remodeling of the lectin/EGF-like interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force. Nature Immunol. 7, 883–889 (2006)
Astrof, N. S., Salas, A., Shimaoka, M., Chen, J. F. & Springer, T. A. Importance of force linkage in mechanochemistry of adhesion receptors. Biochemistry 45, 15020–15028 (2006)
Springer, T. A. Structural basis for selectin mechanochemistry. Proc. Natl Acad. Sci. USA 106, 91–96 (2009)
Thomas, W. E., Vogel, V. & Sokurenko, E. Biophysics of catch bonds. Annu Rev Biophys 37, 399–416 (2008)
Schneider, S. W. et al. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc. Natl Acad. Sci. USA 104, 7899–7903 (2007)
Zhang, X., Halvorsen, K., Zhang, C. Z., Wong, W. P. & Springer, T. A. Mechanoenzymatic cleavage of the ultralarge vascular protein, von Willebrand Factor. Science 324, 1330–1334 (2009)
Yago, T. et al. Platelet glycoprotein Ibα forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J. Clin. Invest. 118, 3195–3207 (2008)
Ulrichts, H. et al. Shielding of the A1 domain by the D′D3 domains of von Willebrand factor modulates its interaction with platelet glycoprotein Ib-IX-V. J. Biol. Chem. 281, 4699–4707 (2006)
Chen, S. & Springer, T. A. An automatic braking system that stabilizes leukocyte rolling by an increase in selectin bond number with shear. J. Cell Biol. 144, 185–200 (1999)
Shen, Y. et al. Requirement of leucine-rich repeats of glycoprotein (GP) Ibα for shear-dependent and static binding of von Willebrand factor to the platelet membrane GP Ib-IX-V complex. Blood 95, 903–910 (2000)
Shen, Y. et al. Leucine-rich repeats 2-4 (Leu60-Glu128) of platelet glycoprotein Ibα regulate shear-dependent cell adhesion to von Willebrand factor. J. Biol. Chem. 281, 26419–26423 (2006)
Celikel, R., Ruggeri, Z. M. & Varughese, K. I. von Willebrand factor conformation and adhesive function is modulated by an internalized water molecule. Nature Struct. Biol. 7, 881–884 (2000)
Kieliszewski, M. J., Leykam, J. F. & Lamport, D. T. Trypsin cleaves lysylproline in a hydroxyproline-rich glycoprotein from Zea mays. Pept. Res. 2, 246–248 (1989)
Smith, S. B., Cui, Y. & Bustamante, C. Optical-trap force transducer that operates by direct measurement of light momentum. Methods Enzymol. 361, 134–162 (2003)
Thomas, W. et al. Catch-bond model derived from allostery explains force-activated bacterial adhesion. Biophys. J. 90, 753–764 (2006)
Aricescu, A. R., Lu, W. & Jones, E. Y. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D 62, 1243–1250 (2006)
Acknowledgements
Supported by NIH HL-48675 (TAS). The authors are indebted to S. B. Smith and C. Bustamante for help with laser tweezers construction and insightful discussion. We thank B. Coller, O. K. Dudko and C. Lu for reagents and insightful discussions, and J. Dill for software for data analysis.
Author information
Authors and Affiliations
Contributions
T.A.S. designed and supervised the project. X.Z. cloned the ReaLiSM construct. J.K. designed experiments and collected and analysed data. C.-Z.Z. analysed data. T.A.S, J.K. and C.-Z.Z. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Results, Supplementary Figures 1- 6 with legends, Supplementary Tables 1-2 and References. (PDF 774 kb)
Rights and permissions
About this article
Cite this article
Kim, J., Zhang, CZ., Zhang, X. et al. A mechanically stabilized receptor–ligand flex-bond important in the vasculature. Nature 466, 992–995 (2010). https://doi.org/10.1038/nature09295
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature09295
This article is cited by
-
A computational investigation of occlusive arterial thrombosis
Biomechanics and Modeling in Mechanobiology (2024)
-
Biomechanical activation of blood platelets via adhesion to von Willebrand factor studied with mesoscopic simulations
Biomechanics and Modeling in Mechanobiology (2023)
-
A Multiscale Model for Shear-Mediated Platelet Adhesion Dynamics: Correlating In Silico with In Vitro Results
Annals of Biomedical Engineering (2023)
-
The role of single-protein elasticity in mechanobiology
Nature Reviews Materials (2022)
-
Weak catch bonds make strong networks
Nature Materials (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.