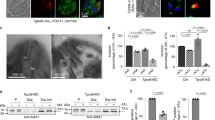
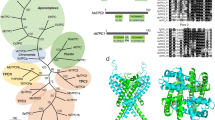
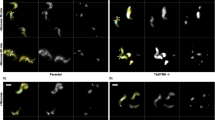
Abstract
Calcium-regulated exocytosis is a ubiquitous process in eukaryotes, whereby secretory vesicles fuse with the plasma membrane and release their contents in response to an intracellular calcium surge1. This process regulates various cellular functions such as plasma membrane repair in plants and animals2,3, the discharge of defensive spikes in Paramecium4, and the secretion of insulin from pancreatic cells, immune modulators from lymphocytes, and chemical transmitters from neurons5. In animal cells, serine/threonine kinases including cAMP-dependent protein kinase, protein kinase C and calmodulin kinases have been implicated in calcium-signal transduction leading to regulated secretion1,6,7. Although plants and protozoa also regulate secretion by means of intracellular calcium, the method by which these signals are relayed has not been explained. Here we show that the Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) is an essential regulator of calcium-dependent exocytosis in this opportunistic human pathogen. Conditional suppression of TgCDPK1 revealed that it controls calcium-dependent secretion of specialized organelles called micronemes, resulting in a block of essential phenotypes including parasite motility, host-cell invasion, and egress. These phenotypes were recapitulated by using a chemical biology approach in which pyrazolopyrimidine-derived compounds specifically inhibited TgCDPK1 and disrupted the parasite’s life cycle at stages dependent on microneme secretion. Inhibition was specific to TgCDPK1, because expression of a resistant mutant kinase reversed sensitivity to the inhibitor. TgCDPK1 is conserved among apicomplexans and belongs to a family of kinases shared with plants and ciliates8, suggesting that related CDPKs may have a function in calcium-regulated secretion in other organisms. Because this kinase family is absent from mammalian hosts, it represents a validated target that may be exploitable for chemotherapy against T. gondii and related apicomplexans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Barclay, J. W., Morgan, A. & Burgoyne, R. D. Calcium-dependent regulation of exocytosis. Cell Calcium 38, 343–353 (2005)
Schapire, A. L., Valpuesta, V. & Botella, M. A. Plasma membrane repair in plants. Trends Plant Sci. 14, 645–652 (2009)
Bansal, D. & Campbell, K. P. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol. 14, 206–213 (2004)
Vayssie, L., Skouri, F., Sperling, L. & Cohen, J. Molecular genetics of regulated secretion in paramecium. Biochimie 82, 269–288 (2000)
Chieregatti, E. & Meldolesi, J. Regulated exocytosis: new organelles for non-secretory purposes. Nature Rev. Mol. Cell Biol. 6, 181–187 (2005)
Choi, W. S., Chahdi, A., Kim, Y. M., Fraundorfer, P. F. & Beaven, M. A. Regulation of phospholipase D and secretion in mast cells by protein kinase A and other protein kinases. Ann. NY Acad. Sci. 968, 198–212 (2002)
Easom, R. A. CaM kinase II: a protein kinase with extraordinary talents germane to insulin exocytosis. Diabetes 48, 675–684 (1999)
Billker, O., Lourido, S. & Sibley, L. D. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe 5, 612–622 (2009)
Carruthers, V. B. & Sibley, L. D. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii . Mol. Microbiol. 31, 421–428 (1999)
Carruthers, V. B., Giddings, O. K. & Sibley, L. D. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell. Microbiol. 1, 225–236 (1999)
Harper, J. F. & Harmon, A. C. Plants, symbiosis and parasites: a calcium signalling connection. Nature Rev. Mol. Cell Biol. 6, 555–566 (2005)
Wernimont, A. et al. Structural analysis of calcium-dependent protein kinases reveal mechanism of activation by calcium. Nature Struct. Mol. Biol. 10.1038/nsmb.1795 (2 May 2010)
Billker, O. et al. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell 117, 503–514 (2004)
Kieschnick, H., Wakefield, T., Narducci, C. A. & Beckers, C. Toxoplasma gondii attachment to host cells is regulated by a calmodulin- like domain protein kinase. J. Biol. Chem. 276, 12369–12377 (2001)
Meissner, M., Schluter, D. & Soldati, D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298, 837–840 (2002)
Sibley, L. D. Invasion strategies of intracellular parasites. Science 304, 248–253 (2004)
Nagamune, K. et al. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii . Nature 451, 207–211 (2008)
Endo, T., Sethi, K. K. & Piekarski, G. Toxoplasma gondii: calcium ionophore A23187-mediated exit of trophozoites from infected murine macrophages. Exp. Parasitol. 53, 179–188 (1982)
Carruthers, V. B., Sherman, G. D. & Sibley, L. D. The Toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J. Biol. Chem. 275, 14346–14353 (2000)
Carruthers, V. B., Moreno, S. N. J. & Sibley, L. D. Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii . Biochem. J. 342, 379–386 (1999)
Kafsack, B. F. et al. Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science 323, 530–533 (2009)
Bishop, A. C. et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407, 395–401 (2000)
Sugi, T. et al. Use of the kinase inhibitor analog 1NM-PP1 reveals a role for Toxoplasma gondii CDPK1 in the invasion step. Eukaryot. Cell 9, 667–670 (2010)
Gurnett, A. M. et al. Purification and molecular characterization of cGMP-dependent protein kinase from Apicomplexan parasites. A novel chemotherapeutic target. J. Biol. Chem. 277, 15913–15922 (2002)
Wiersma, H. I. et al. A role for coccidian cGMP-dependent protein kinase in motility and invasion. Int. J. Parasitol. 34, 369–380 (2004)
Starnes, G. L., Coincon, M., Sygusch, J. & Sibley, L. D. Aldolase is essential for energy production and bridging adhesin–actin cytoskeletal interactions during parasite invasion of host cells. Cell Host Microbe 5, 353–364 (2009)
Roos, D. S., Donald, R. G. K., Morrissette, N. S. & Moulton, A. L. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii . Methods Cell Biol. 45, 28–61 (1994)
Huynh, M. H. et al. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2–M2AP adhesive protein complex. EMBO J. 22, 2082–2090 (2003)
Kim, K., Soldati, D. & Boothroyd, J. C. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science 262, 911–914 (1993)
Messina, M., Niesman, I. R., Mercier, C. & Sibley, L. D. Stable DNA transformation of Toxoplasma gondii using phleomycin selection. Gene 165, 213–217 (1995)
Donald, R. G. K. & Roos, D. S. Stable molecular transformation of Toxoplasma gondii: a selectable dihydrofolate reductase–thymidylate synthase marker based on drug resistance mutations in malaria. Proc. Natl Acad. Sci. USA 90, 11703–11707 (1993)
Håkansson, S., Morisaki, H., Heuser, J. E. & Sibley, L. D. Time-lapse video microscopy of gliding motility in Toxoplasma gondii reveals a novel, biphasic mechanism of cell locomotion. Mol. Biol. Cell 10, 3539–3547 (1999)
Blair, J. A. et al. Structure-guided development of affinity probes for tyrosine kinases using chemical genetics. Nature Chem. Biol. 3, 229–238 (2007)
Acknowledgements
We thank O. Billker, G. Ward, S. Moreno and V. Carruthers for discussions; F. Dzierszinski for the DsRed plasmid; D. Soldati for the Tet-transactivator system; and K. Tang for technical assistance. This work was supported by a predoctoral fellowship from the American Heart Association (S.L.) and a grant from the National Institutes of Health (L.D.S.).
Author information
Authors and Affiliations
Contributions
S.L. designed and performed the majority of experiments, analysed the data, generated the figures and wrote the manuscript. J.S. performed the video miscopy measurements of motility and analysed the data. R.H. provided key insight into the regulation of CDPKs by calcium. C.Z. and K.M.S. provided inhibitors and insight into the strategy for chemical biology experiments. L.D.S. supervised the project, assisted with experimental design and analyses, and contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-4 with legends and Supplementary Tables 1-2. (PDF 3880 kb)
Supplementary Movie 1
This movie shows ionophore-tiggered egress of cKO parasites grown in the absence of ATc. Groups of intracellular parasites are observed in round vacuoles. The time lapse covers a period of about 5 min during which time parasites start moving and egress normally from the host cells. Time stamp is given as h:min:sec. (MOV 3868 kb)
Supplementary Movie 2
This movie shows cKO vacuoles grown in the presence of ATc fail to egress upon ionophore treatment. Independent parasite vacuoles in five host cells can be observed. The time lapse covers a period of about 5 min, during which time wild type parasites would have normally egressed from the host cells, but the TgCDPK1 depleted parasites remain intracellular. Time stamp is given as h:min:sec. (MOV 2858 kb)
Supplementary Movie 3
This movie shows PVM permeabilization by DsRed-expressing WT parasites grown in the presence of ATc. Six round parasite vacuoles are shown in three independent host cells. Parasites were treated with ionophore immediately prior to recording, and immobilization with cytochalasin D prevented mechanical rupture by the PVM. The time lapse covers a period of about 9 min during which time DsRed is released from all vacuoles into their respective host cells. Time stamp is given as h:min:sec. (MOV 561 kb)
Supplementary Movie 4
This movie shows PVM permeabilization by DsRed-expressing cKO parasites grown in the presence of ATc. The time lapse covers a period of about 9 min during which time only one of the two of vacuoles releases DsRed at a much slower rate than WT. Time stamp is given as h:min:sec. (MOV 524 kb)
Rights and permissions
About this article
Cite this article
Lourido, S., Shuman, J., Zhang, C. et al. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature 465, 359–362 (2010). https://doi.org/10.1038/nature09022
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature09022
This article is cited by
-
Calreticulin (CALR) promotes ionophore-induced microneme secretion in Toxoplasma gondii
Parasitology Research (2024)
-
Rapid metabolic reprogramming mediated by the AMP-activated protein kinase during the lytic cycle of Toxoplasma gondii
Nature Communications (2023)
-
Effect of deleting four Toxoplasma gondii calcium-binding EGF domain-containing proteins on parasite replication and virulence
Parasitology Research (2023)
-
Screening the Toxoplasma kinome with high-throughput tagging identifies a regulator of invasion and egress
Nature Microbiology (2022)
-
Live-attenuated ME49Δcdpk3 strain of Toxoplasma gondii protects against acute and chronic toxoplasmosis
npj Vaccines (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.