Abstract
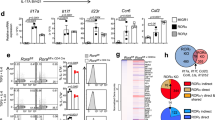
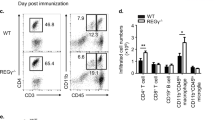
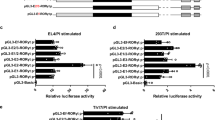
Interleukin (IL)-17-producing helper T (TH17) cells are a distinct T-cell subset characterized by its pathological role in autoimmune diseases1,2,3. IL-6 and transforming growth factor-β (TGF-β) induce TH17 development, in which the orphan nuclear receptors, RORγt and RORα, have an indispensable role4,5,6. However, in the absence of IL-6 and TGF-β, the ectopic expression of RORγt or RORα leads to only a modest IL-17 production5,7,8. Here we identify a nuclear IκB family member, IκBζ (encoded by the Nfkbiz gene), as a transcription factor required for TH17 development in mice. The ectopic expression of IκBζ in naive CD4+ T cells together with RORγt or RORα potently induces TH17 development, even in the absence of IL-6 and TGF-β. Notably, Nfkbiz-/- mice have a defect in TH17 development and a resistance to experimental autoimmune encephalomyelitis (EAE). The T-cell-intrinsic function of IκBζ was clearly demonstrated by the resistance to EAE of the Rag2-/- mice into which Nfkbiz-/- CD4+ T cells were transferred. In cooperation with RORγt and RORα, IκBζ enhances Il17a expression by binding directly to the regulatory region of the Il17a gene. This study provides evidence for the transcriptional mechanisms underlying TH17 development and points to a molecular basis for a novel therapeutic strategy against autoimmune disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 (2009)
Dong, C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nature Rev. Immunol. 8, 337–348 (2008)
O’Shea, J. J. et al. Signal transduction and Th17 cell differentiation. Microbes Infect. 11, 599–611 (2009)
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006)
Yang, X. O. et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity 28, 29–39 (2008)
Manel, N., Unutmaz, D. & Littman, D. R. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nature Immunol. 9, 641–649 (2008)
Brüstle, A. et al. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nature Immunol. 8, 958–966 (2007)
Huber, M. et al. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc. Natl Acad. Sci. USA 105, 20846–20851 (2008)
Yamazaki, S., Muta, T. & Takeshige, K. A novel IκB protein, IκB-ζ, induced by proinflammatory stimuli, negatively regulates nuclear factor-κB in the nuclei. J. Biol. Chem. 276, 27657–27662 (2001)
Muta, T. IκB-ζ: an inducible regulator of nuclear factor-κB. Vitam. Horm. 74, 301–316 (2006)
Yamamoto, M. et al. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IκBζ. Nature 430, 218–222 (2004)
Yamamoto, M. & Takeda, K. Role of nuclear IκB proteins in the regulation of host immune responses. J. Infect. Chemother. 14, 265–269 (2008)
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006)
Schraml, B. U. et al. The AP-1 transcription factor Batf controls TH17 differentiation. Nature 460, 405–409 (2009)
Quintana, F. J. et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008)
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 (2008)
Zhang, F., Meng, G. & Strober, W. Interactions among the transcription factors Runx1, RORγt and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nature Immunol. 9, 1297–1306 (2008)
Chen, Z. et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl Acad. Sci. USA 103, 8137–8142 (2006)
Motoyama, M., Yamazaki, S., Eto-Kimura, A., Takeshige, K. & Muta, T. Positive and negative regulation of nuclear factor-κB-mediated transcription by IκB-ζ, an inducible nuclear protein. J. Biol. Chem. 280, 7444–7451 (2005)
Yamazaki, S., Muta, T., Matsuo, S. & Takeshige, K. Stimulus-specific induction of a novel nuclear factor-κB regulator, IκB-ζ, via Toll/interleukin-1 receptor is mediated by mRNA stabilization. J. Biol. Chem. 280, 1678–1687 (2005)
Jetten, A. M. & Joo, J. H. Retinoid-related orphan receptors (RORs): roles in cellular differentiation and development. Adv. Dev. Biol. 16, 313–355 (2006)
Matsuo, S., Yamazaki, S., Takeshige, K. & Muta, T. Crucial roles of binding sites for NF-κB and C/EBPs in IκB-ζ-mediated transcriptional activation. Biochem. J. 405, 605–615 (2007)
Akimzhanov, A. M., Yang, X. O. & Dong, C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J. Biol. Chem. 282, 5969–5972 (2007)
Shiina, T. et al. Targeted disruption of MAIL, a nuclear IκB protein, leads to severe atopic dermatitis-like disease. J. Biol. Chem. 279, 55493–55498 (2004)
Kurebayashi, S. et al. Retinoid-related orphan receptorγ (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl Acad. Sci. USA 97, 10132–10137 (2000)
Adachi, O. et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 (1998)
Nakae, S. et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387 (2002)
Sha, W. C., Liou, H. C., Tuomanen, E. I. & Baltimore, D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell 80, 321–330 (1995)
Hamilton, B. A. et al. Disruption of the nuclear hormone receptor RORα in staggerer mice. Nature 379, 736–739 (1996)
Takeda, K. et al. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 161, 4652–4660 (1998)
Asagiri, M. et al. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science 319, 624–627 (2008)
Kitamura, T. et al. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp. Hematol. 31, 1007–1014 (2003)
Shinohara, M. et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell 132, 794–806 (2008)
Koga, T. et al. NFAT and Osterix cooperatively regulate bone formation. Nature Med. 11, 880–885 (2005)
Loots, G. G. & Ovcharenko, I. rVISTA 2.0: evolutionary analysis of transcription factor binding sites. Nucleic Acids Res. 32, W217–W221 (2004)
Acknowledgements
We are grateful to Y. Iwakura and T. Kitamura for providing Il17a-/- mice and retrovirus vectors, respectively. We also thank M. Shinohara, T. Negishi-Koga, M. Asagiri, T. Nakashima, N. Komatsu, M. Ohba, Y. Kunisawa, Y. Suzuki, S. Miyakoshi and T. Kunigami for discussion and assistance. This work was supported in part by Grant-in-Aid for Creative Scientific Research from the Japan Society for the Promotion of Science (JSPS), Grant-in-Aid for Challenging Exploratory Research from JSPS, Grant-in-Aid for JSPS Fellows, Grants-in-Aid for GCOE Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), and ERATO, Takayanagi Osteonetwork Project from JST. It was also supported by grants from the Intramural Research Program of the NIEHS (Z01-ES-101586) (to A.M.J.), Takeda Life Science Foundation and Yokoyama Foundation for Clinical Pharmacology and the Ichiro Kanehara Foundation. Ka.O. is supported by JSPS Research Fellowships for Young Scientists.
Author information
Authors and Affiliations
Contributions
Ka.O. performed all of the experiments, interpreted the results and prepared the manuscript. Y.I. contributed to dendritic cells experiments and T-cell transfer experiments. M.O. contributed to study design and manuscript preparation. M.Y., A.M.J. and S.A. provided genetically modified mice and advice on data analysis. To.M. provided advice on project planning and data interpretation. K.A. and Ke.O. supported the experiments using Nfkb1-/- mice. Ta.M. provided genetically modified mice and the plasmids, and advised on project planning. H.T. directed the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1–19 with legends, Supplementary Tables 1–2 and Supplementary References. (PDF 2622 kb)
Rights and permissions
About this article
Cite this article
Okamoto, K., Iwai, Y., Oh-hora, M. et al. IκBζ regulates TH17 development by cooperating with ROR nuclear receptors. Nature 464, 1381–1385 (2010). https://doi.org/10.1038/nature08922
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08922
This article is cited by
-
IκBζ controls IL-17-triggered gene expression program in intestinal epithelial cells that restricts colonization of SFB and prevents Th17-associated pathologies
Mucosal Immunology (2022)
-
Metabolic regulation and function of T helper cells in neuroinflammation
Seminars in Immunopathology (2022)
-
NF-κB in control of regulatory T cell development, identity, and function
Journal of Molecular Medicine (2022)
-
Itaconate inhibits TET DNA dioxygenases to dampen inflammatory responses
Nature Cell Biology (2022)
-
Aging-associated lncRNAs are evolutionarily conserved and participate in NFκB signaling
Nature Aging (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.