Abstract
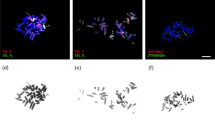
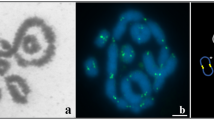
Although bisexual reproduction has proven to be highly successful, parthenogenetic all-female populations occur frequently in certain taxa, including the whiptail lizards of the genus Aspidoscelis. Allozyme analysis revealed a high degree of fixed heterozygosity in these parthenogenetic species1,2, supporting the view that they originated from hybridization events between related sexual species. It has remained unclear how the meiotic program is altered to produce diploid eggs while maintaining heterozygosity. Here we show that meiosis commences with twice the number of chromosomes in parthenogenetic versus sexual species, a mechanism that provides the basis for generating gametes with unreduced chromosome content without fundamental deviation from the classic meiotic program. Our observation of synaptonemal complexes and chiasmata demonstrate that a typical meiotic program occurs and that heterozygosity is not maintained by bypassing recombination. Instead, fluorescent in situ hybridization probes that distinguish between homologues reveal that bivalents form between sister chromosomes, the genetically identical products of the first of two premeiotic replication cycles. Sister chromosome pairing provides a mechanism for the maintenance of heterozygosity, which is critical for offsetting the reduced fitness associated with the lack of genetic diversity in parthenogenetic species.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Neaves, W. B. Adenosine deaminase phenotypes among sexual and parthenogenetic lizards in the genus Cnemidophorus (Teiidae). J. Exp. Zool. 171, 175–183 (1969)
Neaves, W. B. & Gerald, P. S. Lactate dehydrogenase isozymes in parthenogenetic teiid lizards (Cnemidophorus). Science 160, 1004–1005 (1968)
Vrijenhoek, R. C., Dawley, R. M., Cole, C. J. & Bogart, J. P. in Evolution and Ecology of Unisexual Vertebrates Vol. 466 (eds Dawley, R. M. & Bogart, J. P.) 19–23 (New York State Museum Bulletin, 1989)
Reeder, T. W., Cole, C. J. & Dessauer, H. C. Phylogenetic relationships of whiptail lizards of the genus Cnemidophorus (Squamata: Teiidae): a test of monophyly, reevaluation of karyotypic evolution, and review of hybrid origins. Am. Mus. Novit. 3365, 1–61 (2002)
Lowe, C. H. & Wright, J. W. Evolution of parthenogenetic species of Cnemidophorus (whiptail lizards) in western North America. J. Ariz. Acad. Sci. 4, 81–87 (1966)
Dessauer, H. C. & Cole, C. J. Clonal inheritance in parthenogenetic whiptail lizards: biochemical evidence. J. Hered. 77, 8–12 (1986)
Cordes, J. E. & Walker, J. M. Skin histocompatibility between syntopic pattern classes C and D of parthenogenetic Cnemidophorus tesselatus in New Mexico. J. Herpetol. 37, 185–188 (2003)
Cordes, J. E. & Walker, J. M. Evolutionary and systematic implications of skin histocompatibility among parthenogenetic teiid lizards: three color pattern classes of Aspidoscelis dixoni and one of Aspidoscelis tesselata . Copeia 14–26 (2006)
Maslin, T. P. Skin grafting in the bisexual teiid lizard Cnemidophorus sexlineatus and in the unisexual C. tesselatus . J. Exp. Zool. 166, 137–149 (1967)
Cuellar, O. & Smart, C. Analysis of histoincompatibility in a natural population of the bisexual whiptail lizard Cnemidophorus tigris . Transplantation 24, 127–133 (1977)
Cuellar, O. Reproduction and the mechanism of meiotic restitution in the parthenogenetic lizard Cnemidophorus uniparens . J. Morphol. 133, 139–165 (1971)
Neaves, W. B. Tetraploidy in a hybrid lizard of the genus Cnemidophorus (Teiidae). Brev. Mus. Comp. Zool. 381, 1–25 (1971)
Dessauer, H. C. & Cole, C. J. in Evolution and Ecology of Unisexual Vertebrates Vol. 466 (eds Dawley, R. M. & Bogard, J. P.) 49–71 (New York State Museum Bulletin, 1989)
Parker, E. D. & Selander, R. K. The organization of genetic diversity in the parthenogenetic lizard Cnemidophorus tesselatus . Genetics 84, 791–805 (1976)
Wright, J. W. & Lowe, C. H. Evolution of the alloploid parthenospecies Cnemidophorus tesselatus (Say). Mamm. Chromosome Newsl. 8, 95–96 (1967)
Edgar, B. A. & Orr-Weaver, T. L. Endoreplication cell cycles: more for less. Cell 105, 297–306 (2001)
Macgregor, H. C. & Uzzell, T. M. Gynogenesis in salamanders related to Ambystoma jeffersonianum . Science 143, 1043–1045 (1964)
White, M. J. D., Cheney, J. & Key, K. H. L. A parthenogenetic species of grasshopper with complex structural heterozygosity (Orthoptera: Acridoidea). Aust. J. Zool. 11, 1–19 (1963)
Townsend, C. R. Establishment and maintenance of colonies of parthenogenetic whiptail lizards: Cnemidophorus spp. Int. Zoo. Yb. 19, 80–86 (1979)
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990)
Otsu, N. Threshold selection method from gray-level-histograms. IEEE Trans. Syst. Man Cybern. SMC-9, 62–66 (1979)
Gonzalez, R. C. & Woods, R. E. Digital Image Processing 3rd edn, (Pearson/Prentice Hall, 2008)
ImageJ. (US National Institutes of Health, Bethesda,, 1997–2009)
Moore, M. K., Work, T. M., Balazs, G. H. & Docherty, D. E. Preparation, cryopreservation, and growth of cells prepared from the green turtle (Chelonia mydas). Methods Cell Sci. 19, 161–168 (1997)
Sarthy, J., Bae, N. S., Scrafford, J. & Baumann, P. Human RAP1 inhibits non-homologous end joining at telomeres. EMBO J. 28, 3390–3399 (2009)
Acknowledgements
We thank the staff of the Reptile Facility for their dedication to animal husbandry; C. Painter and his colleagues at the New Mexico Department of Game and Fish for scientific collection permits; F. Li for electron microscopy; the Stowers Institute Microscopy, Cytometry and Molecular Biology Facilities for support; and S. Hawley, J. Cole, C. Townsend and members of the Baumann and Blanchette laboratories for discussions and comments. We also thank S. Hawley for critical reading of the manuscript. This work was funded by the Stowers Institute for Medical Research, and the Pew Scholars Program in the Biological Sciences sponsored by the Pew Charitable Trusts. P.B. is an Early Career Scientist with the Howard Hughes Medical Institute.
Author Contributions A.A.L., W.B.N., D.P.B. and P.B. contributed extensively to the work presented in this paper. W.W. performed, and provided training in, microscopy and image data quantification.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-3 with Legends and Supplementary Table 1. (PDF 1933 kb)
Supplementary Movie 1
This movie file shows an animation of the rendered chromosomes shown in Supplementary Figure 1b (see Supplementary Information file). (AVI 4740 kb)
Rights and permissions
About this article
Cite this article
Lutes, A., Neaves, W., Baumann, D. et al. Sister chromosome pairing maintains heterozygosity in parthenogenetic lizards. Nature 464, 283–286 (2010). https://doi.org/10.1038/nature08818
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08818
This article is cited by
-
Evolutionary mechanisms and practical significance of reproductive success and clonal diversity in unisexual vertebrate polyploids
Science China Life Sciences (2024)
-
Sex determination mechanisms and sex control approaches in aquaculture animals
Science China Life Sciences (2022)
-
Genotypic similarities among the parthenogenetic Darevskia rock lizards with different hybrid origins
BMC Evolutionary Biology (2020)
-
Origin, clonal diversity, and evolution of the parthenogenetic lizard Darevskia unisexualis
BMC Genomics (2020)
-
Cytogenetic mechanisms of unisexuality in rock lizards
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.