Abstract
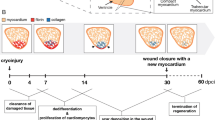
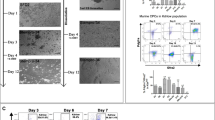
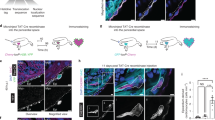
Recent studies indicate that mammals, including humans, maintain some capacity to renew cardiomyocytes throughout postnatal life1,2. Yet, there is little or no significant cardiac muscle regeneration after an injury such as acute myocardial infarction3. By contrast, zebrafish efficiently regenerate lost cardiac muscle, providing a model for understanding how natural heart regeneration may be blocked or enhanced4,5. In the absence of lineage-tracing technology applicable to adult zebrafish, the cellular origins of newly regenerated cardiac muscle have remained unclear. Using new genetic fate-mapping approaches, here we identify a population of cardiomyocytes that become activated after resection of the ventricular apex and contribute prominently to cardiac muscle regeneration. Through the use of a transgenic reporter strain, we found that cardiomyocytes throughout the subepicardial ventricular layer trigger expression of the embryonic cardiogenesis gene gata4 within a week of trauma, before expression localizes to proliferating cardiomyocytes surrounding and within the injury site. Cre-recombinase-based lineage-tracing of cells expressing gata4 before evident regeneration, or of cells expressing the contractile gene cmlc2 before injury, each labelled most cardiac muscle in the ensuing regenerate. By optical voltage mapping of surface myocardium in whole ventricles, we found that electrical conduction is re-established between existing and regenerated cardiomyocytes between 2 and 4 weeks post-injury. After injury and prolonged fibroblast growth factor receptor inhibition to arrest cardiac regeneration and enable scar formation, experimental release of the signalling block led to gata4 expression and morphological improvement of the injured ventricular wall without loss of scar tissue. Our results indicate that electrically coupled cardiac muscle regenerates after resection injury, primarily through activation and expansion of cardiomyocyte populations. These findings have implications for promoting regeneration of the injured human heart.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bergmann, O. et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009)
Quaini, F. et al. Chimerism of the transplanted heart. N. Engl. J. Med. 346, 5–15 (2002)
Rubart, M. & Field, L. J. Cardiac regeneration: repopulating the heart. Annu. Rev. Physiol. 68, 29–49 (2006)
Poss, K. D., Wilson, L. G. & Keating, M. T. Heart regeneration in zebrafish. Science 298, 2188–2190 (2002)
Raya, A. et al. Activation of notch signaling pathway precedes heart regeneration in zebrafish. Proc. Natl Acad. Sci. USA 100, 11889–11895 (2003)
Cai, C. L. et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 5, 877–889 (2003)
Dor, Y., Brown, J., Martinez, O. I. & Melton, D. A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 (2004)
Hsieh, P. C. et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nature Med. 13, 970–974 (2007)
Laugwitz, K. L. et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 433, 647–653 (2005)
Meilhac, S. M. et al. A retrospective clonal analysis of the myocardium reveals two phases of clonal growth in the developing mouse heart. Development 130, 3877–3889 (2003)
Zhou, B. et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454, 109–113 (2008)
Holtzinger, A. & Evans, T. Gata4 regulates the formation of multiple organs. Development 132, 4005–4014 (2005)
Crispino, J. D. et al. Proper coronary vascular development and heart morphogenesis depend on interaction of GATA-4 with FOG cofactors. Genes Dev. 15, 839–844 (2001)
Molkentin, J. D., Lin, Q., Duncan, S. A. & Olson, E. N. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 11, 1061–1072 (1997)
Kuo, C. T. et al. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 11, 1048–1060 (1997)
Zeisberg, E. M. et al. Morphogenesis of the right ventricle requires myocardial expression of Gata4 . J. Clin. Invest. 115, 1522–1531 (2005)
Pu, W. T., Ishiwata, T., Juraszek, A. L., Ma, Q. & Izumo, S. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev. Biol. 275, 235–244 (2004)
Heicklen-Klein, A. & Evans, T. T-box binding sites are required for activity of a cardiac GATA-4 enhancer. Dev. Biol. 267, 490–504 (2004)
Lepilina, A. et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 127, 607–619 (2006)
Laflamme, M. A., Zbinden, S., Epstein, S. E. & Murry, C. E. Cell-based therapy for myocardial ischemia and infarction: pathophysiological mechanisms. Annu. Rev. Pathol. 2, 307–339 (2007)
Passier, R., van Laake, L. W. & Mummery, C. L. Stem-cell-based therapy and lessons from the heart. Nature 453, 322–329 (2008)
de Pater, E. et al. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development 136, 1633–1641 (2009)
Bersell, K., Arab, S., Haring, B. & Kuhn, B. Neuregulin1/erbb4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138, 257–270 (2009)
Kühn, B. et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nature Med. 13, 962–969 (2007)
Engel, F. B., Hsieh, P. C., Lee, R. T. & Keating, M. T. Fgf1/p38 map kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc. Natl Acad. Sci. USA 103, 15546–15551 (2006)
Engel, F. B. et al. p38 map kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 19, 1175–1187 (2005)
Wills, A. A., Holdway, J. E., Major, R. J. & Poss, K. D. Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development 135, 183–192 (2008)
Matsuda, T. & Cepko, C. L. Controlled expression of transgenes introduced by in vivo electroporation. Proc. Natl Acad. Sci. USA 104, 1027–1032 (2007)
Thermes, V. et al. I-scei meganuclease mediates highly efficient transgenesis in fish. Mech. Dev. 118, 91–98 (2002)
Indra, A. K. et al. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-Er(T2) recombinases. Nucleic Acids Res. 27, 4324–4327 (1999)
Rottbauer, W. et al. Reptin and pontin antagonistically regulate heart growth in zebrafish embryos. Cell 111, 661–672 (2002)
Waxman, J. S., Keegan, B. R., Roberts, R. W., Poss, K. D. & Yelon, D. Hoxb5b acts downstream of retinoic acid signaling in the forelimb field to restrict heart field potential in zebrafish. Dev. Cell 15, 923–934 (2008)
Sidorov, V. Y., Holcomb, M. R., Woods, M. C., Gray, R. A. & Wikswo, J. P. Effects of unipolar stimulation on voltage and calcium distributions in the isolated rabbit heart. Basic Res. Cardiol. 103, 537–551 (2008)
Brette, F. et al. Characterization of isolated ventricular myocytes from adult zebrafish (Danio Rerio). Biochem. Biophys. Res. Commun. 374, 143–146 (2008)
Fast, V. G. & Kleber, A. G. Cardiac tissue geometry as a determinant of unidirectional conduction block: assessment of microscopic excitation spread by optical mapping in patterned cell cultures and in a computer model. Cardiovasc. Res. 29, 697–707 (1995)
Bayly, P. V. et al. Estimation of conduction velocity vector fields from epicardial mapping data. IEEE Trans. Biomed. Eng. 45, 563–571 (1998)
Acknowledgements
We thank J. Burris and A. Eastes for zebrafish care, X. Meng and the Developmental Studies Hybridoma Bank for antibodies, M. Gignac for help with electron microscopy, laboratory members for comments on the manuscript, and G. Burns, P. Chambon and G. Felsenfeld for plasmids. This work was supported by postdoctoral fellowships from AHA (K.K. and Y.F.), JDRF (R.M.A.), and JSPS (K.K.); NIH training grants HL007208 at Massachusetts General Hospital (A.A.W.) and HL007101 at Duke University Medical Center (G.F.E.); grants from NHLBI (HL064282 to T.E., HL054737 to D.Y.R.S., and HL081674 to K.D.P.), NIGMS (GM075846 to C.A.M.), and March of Dimes (C.A.M.); and grants from AHA, Pew Charitable Trusts and Whitehead Foundation (K.D.P.).
Author Contributions K.K. and K.D.P. designed experimental strategy, analysed data, and prepared the manuscript. K.K., J.E.H. and Y.F. generated and characterized transgenic lines for lineage-tracing. R.M.A. and D.Y.R.S. provided unpublished reagents for lineage-tracing. K.K., J.E.H. and K.D.P. performed regeneration experiments. J.E.H. performed electron microscopy. A.A.W., G.F.E. and C.A.M. designed physiology experiments and interpreted data. A.A.W. performed optical mapping assays. T.E. helped design strategy and provided key reagents. All authors commented on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-10 with legends. (PDF 17172 kb)
Rights and permissions
About this article
Cite this article
Kikuchi, K., Holdway, J., Werdich, A. et al. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature 464, 601–605 (2010). https://doi.org/10.1038/nature08804
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08804
This article is cited by
-
Animal models to study cardiac regeneration
Nature Reviews Cardiology (2024)
-
Ligament injury in adult zebrafish triggers ECM remodeling and cell dedifferentiation for scar-free regeneration
npj Regenerative Medicine (2023)
-
Fluorescent sensors for imaging of interstitial calcium
Nature Communications (2023)
-
hapln1a+ cells guide coronary growth during heart morphogenesis and regeneration
Nature Communications (2023)
-
Endothelial Brg1 fine-tunes Notch signaling during zebrafish heart regeneration
npj Regenerative Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.