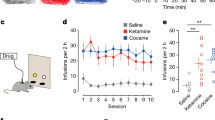
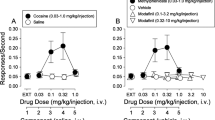
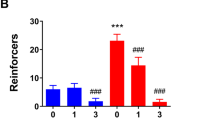
Abstract
Benzodiazepines are widely used in clinics and for recreational purposes, but will lead to addiction in vulnerable individuals. Addictive drugs increase the levels of dopamine and also trigger long-lasting synaptic adaptations in the mesolimbic reward system that ultimately may induce the pathological behaviour. The neural basis for the addictive nature of benzodiazepines, however, remains elusive. Here we show that benzodiazepines increase firing of dopamine neurons of the ventral tegmental area through the positive modulation of GABAA (γ-aminobutyric acid type A) receptors in nearby interneurons. Such disinhibition, which relies on α1-containing GABAA receptors expressed in these cells, triggers drug-evoked synaptic plasticity in excitatory afferents onto dopamine neurons and underlies drug reinforcement. Taken together, our data provide evidence that benzodiazepines share defining pharmacological features of addictive drugs through cell-type-specific expression of α1-containing GABAA receptors in the ventral tegmental area. The data also indicate that subunit-selective benzodiazepines sparing α1 may be devoid of addiction liability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lüscher, C. & Ungless, M. A. The mechanistic classification of addictive drugs. PLoS Med. 3, e437 (2006)
O’Brien, C. P. Benzodiazepine use, abuse, and dependence. J. Clin. Psychiatry 66 (suppl. 2). 28–33 (2005)
Saal, D., Dong, Y., Bonci, A. & Malenka, R. C. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37, 577–582 (2003)
Bellone, C. & Lüscher, C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nature Neurosci. 9, 636–641 (2006)
Argilli, E., Sibley, D. R., Malenka, R. C., England, P. M. & Bonci, A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J. Neurosci. 28, 9092–9100 (2008)
Mameli, M., Balland, B., Lujan, R. & Luscher, C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 317, 530–533 (2007)
Heikkinen, A. E., Moykkynen, T. P. & Korpi, E. R. Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacol. 34, 290–298 (2009)
Johnson, S. W. & North, R. A. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J. Physiol. (Lond.) 450, 455–468 (1992)
Sigel, E., Schaerer, M. T., Buhr, A. & Baur, R. The benzodiazepine binding pocket of recombinant α1β2γ2 γ-aminobutyric acidA receptors: relative orientation of ligands and amino acid side chains. Mol. Pharmacol. 54, 1097–1105 (1998)
Fritschy, J. M. & Mohler, H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol. 359, 154–194 (1995)
Okada, H., Matsushita, N., Kobayashi, K. & Kobayashi, K. Identification of GABAA receptor subunit variants in midbrain dopaminergic neurons. J. Neurochem. 89, 7–14 (2004)
Arendt, R. M., Greenblatt, D. J., Liebisch, D. C., Luu, M. D. & Paul, S. M. Determinants of benzodiazepine brain uptake: lipophilicity versus binding affinity. Psychopharmacology (Berl.) 93, 72–76 (1987)
Rudolph, U. et al. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature 401, 796–800 (1999)
Möhler, H., Benke, D., Mertens, S. & Fritschy, J. M. GABAA-receptor subtypes differing in α-subunit composition display unique pharmacological properties. Adv. Biochem. Psychopharmacol. 47, 41–53 (1992)
McKernan, R. M. et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor alpha1 subtype. Nature Neurosci. 3, 587–592 (2000)
Cameron, D. L., Wessendorf, M. W. & Williams, J. T. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience 77, 155–166 (1997)
Margolis, E. B., Lock, H., Hjelmstad, G. O. & Fields, H. L. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J. Physiol. (Lond.) 577, 907–924 (2006)
Hsia, A. Y., Malenka, R. C. & Nicoll, R. A. Development of excitatory circuitry in the hippocampus. J. Neurophysiol. 79, 2013–2024 (1998)
Poncer, J. C., Durr, R., Gähwiler, B. H. & Thompson, S. M. Modulation of synaptic GABAA receptor function by benzodiazepines in area CA3 of rat hippocampal slice cultures. Neuropharmacology 35, 1169–1179 (1996)
Long, P. et al. Nerve terminal GABAA receptors activate Ca2+/calmodulin-dependent signaling to inhibit voltage-gated Ca2+ influx and glutamate release. J. Biol. Chem. 284, 8726–8737 (2009)
Barberis, A., Mozrzymas, J. W., Ortinski, P. I. & Vicini, S. Desensitization and binding properties determine distinct α1β2γ2 and α3β2γ2 GABAA receptor-channel kinetic behavior. Eur. J. Neurosci. 25, 2726–2740 (2007)
Yee, B. K. et al. A schizophrenia-related sensorimotor deficit links alpha 3-containing GABAA receptors to a dopamine hyperfunction. Proc. Natl Acad. Sci. USA 102, 17154–17159 (2005)
Löw, K. et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science 290, 131–134 (2000)
Robinson, S., Smith, D. M., Mizumori, S. J. & Palmiter, R. D. Firing properties of dopamine neurons in freely moving dopamine-deficient mice: effects of dopamine receptor activation and anesthesia. Proc. Natl Acad. Sci. USA 101, 13329–13334 (2004)
Hyland, B. I., Reynolds, J. N., Hay, J., Perk, C. G. & Miller, R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience 114, 475–492 (2002)
Pilotto, R., Singer, G. & Overstreet, D. Self-injection of diazepam in naive rats: effects of dose, schedule and blockade of different receptors. Psychopharmacology (Berl.) 84, 174–177 (1984)
Fuchs, V., Burbes, E. & Coper, H. The influence of haloperidol and aminooxyacetic acid on etonitazene, alcohol, diazepam and barbital consumption. Drug Alcohol Depend. 14, 179–186 (1984)
Berridge, K. C. & Pecina, S. Benzodiazepines, appetite, and taste palatability. Neurosci. Biobehav. Rev. 19, 121–131 (1995)
Yerbury, R. E. & Cooper, S. J. Novel benzodiazepine receptor ligands: palatable food intake following zolpidem, CGS 17867A, or Ro23–0364, in the rat. Pharmacol. Biochem. Behav. 33, 303–307 (1989)
Morris, H. V., Nilsson, S., Dixon, C. I., Stephens, D. N. & Clifton, P. G. α1- and α2-containing GABAA receptor modulation is not necessary for benzodiazepine-induced hyperphagia. Appetite 52, 675–683 (2009)
Kalivas, P. W., Duffy, P. & Eberhardt, H. Modulation of A10 dopamine neurons by γ-aminobutyric acid agonists. J. Pharmacol. Exp. Ther. 253, 858–866 (1990)
Xi, Z. X. & Stein, E. A. Nucleus accumbens dopamine release modulation by mesolimbic GABAA receptors-an in vivo electrochemical study. Brain Res. 798, 156–165 (1998)
Doherty, M. & Gratton, A. Differential involvement of ventral tegmental GABAA and GABAB receptors in the regulation of the nucleus accumbens dopamine response to stress. Brain Res. 1150, 62–68 (2007)
Grace, A. A. & Bunney, B. S. Paradoxical GABA excitation of nigral dopaminergic cells: indirect mediation through reticulata inhibitory neurons. Eur. J. Pharmacol. 59, 211–218 (1979)
Klitenick, M. A., DeWitte, P. & Kalivas, P. W. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J. Neurosci. 12, 2623–2632 (1992)
Soyka, M., Bottlender, R. & Moller, H. J. Epidemiological evidence for a low abuse potential of zolpidem. Pharmacopsychiatry 33, 138–141 (2000)
Rowlett, J. K., Platt, D. M., Lelas, S., Atack, J. R. & Dawson, G. R. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc. Natl Acad. Sci. USA 102, 915–920 (2005)
Rudolph, U. & Möhler, H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr. Opin. Pharmacol. 6, 18–23 (2005)
Koob, G. F. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry 42 (suppl. 1). S32–S41 (2009)
Lüscher, C. & Bellone, C. Cocaine-evoked synaptic plasticity: a key to addiction? Nature Neurosci. 11, 737–738 (2008)
Mameli, M. et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nature Neurosci. 12, 1036–1041 (2009)
Redish, A. D., Jensen, S. & Johnson, A. A unified framework for addiction: vulnerabilities in the decision process. Behav. Brain Sci. 31, 415–437 (2008)
Labouèbe, G. et al. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nature Neurosci. 10, 1559–1568 (2007)
Zhao, S. et al. Generation of embryonic stem cells and transgenic mice expressing green fluorescence protein in midbrain dopaminergic neurons. Eur. J. Neurosci. 19, 1133–1140 (2004)
Tamamaki, N. et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 467, 60–79 (2003)
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates (Elsevier, 2004)
Grace, A. A. & Bunney, B. S. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—1. Identification and characterization. Neuroscience 10, 301–315 (1983)
Ungless, M. A., Magill, P. J. & Bolam, J. P. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303, 2040–2042 (2004)
Acknowledgements
We thank members of the Lüscher laboratory as well as M. Frerking, M. Serafin and H. Möhler for critical reading of the manuscript. Y. Yanagawa provided the GAD67–GFP Δneo mouse line, and we thank K. A. Miczek and H. U. Zeilhofer for help with the GABAA mutant mouse lines. This work is supported by the National Institute on Drug Abuse (NIDA; DA019022; C.L., P. Slesinger), the Swiss National Science Foundation, the Swiss Initiative in Systems Biology (Neurochoice) and the European Commission Coordination Action ENINET (LSHM-CT-2005-19063). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDA or the National Institutes of Health.
Author Contributions K.R.T. carried out all in vitro electrophysiology experiments. M.B., G.L. and C.Y. contributed equally to the in vivo recordings. K.R.T. and C.C. performed the behavioural experiments. J.-M.F. carried out the immunohistochemistry. U.R. generated the mutant mice. C.L. designed the study and wrote the manuscript with the help of all authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-10 with Legends. (PDF 2647 kb)
Rights and permissions
About this article
Cite this article
Tan, K., Brown, M., Labouèbe, G. et al. Neural bases for addictive properties of benzodiazepines. Nature 463, 769–774 (2010). https://doi.org/10.1038/nature08758
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08758
This article is cited by
-
Alprazolam exposure during adolescence induces long-lasting dysregulation in reward sensitivity to morphine and second messenger signaling in the VTA-NAc pathway
Scientific Reports (2023)
-
Cerebellar α6GABAA Receptors as a Therapeutic Target for Essential Tremor: Proof-of-Concept Study with Ethanol and Pyrazoloquinolinones
Neurotherapeutics (2023)
-
Black-Box Warnings of Antiseizure Medications: What is Inside the Box?
Pharmaceutical Medicine (2023)
-
Activation of mesocorticolimbic dopamine projections initiates cue-induced reinstatement of reward seeking in mice
Acta Pharmacologica Sinica (2022)
-
Dual action of ketamine confines addiction liability
Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.