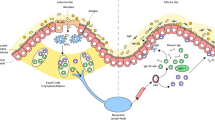
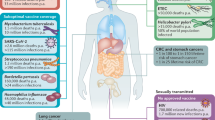
Abstract
Measures to prevent sexual mucosal transmission of human immunodeficiency virus (HIV)-1 are urgently needed to curb the growth of the acquired immunodeficiency syndrome (AIDS) pandemic and ultimately bring it to an end. Studies in animal models and acute HIV-1 infection reviewed here reveal potential viral vulnerabilities at the mucosal portal of entry in the earliest stages of infection that might be most effectively targeted by vaccines and microbicides, thereby preventing acquisition and averting systemic infection, CD4 T-cell depletion and pathologies that otherwise rapidly ensue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
UNAIDS. 2008 Report on the global AIDS epidemic 〈http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp〉 (2008)
Fauci, A. S. 25 years of HIV. Nature 453, 289–290 (2008)
Cohen, J. Treatment and prevention exchange vows at international conference. Science 321, 902–903 (2008)
Bailey, R. C. et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 369, 643–656 (2007)
Auvert, B. et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2, e298 (2005)
Gray, R. H. et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 369, 657–666 (2007)
Rerks-Ngarm, S. et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361, 2209–2220 (2009)
Karim, S. A. et al. Safety and effectiveness of vaginal microbicides BufferGel and 0.5% PRO 2000/5 gel for the prevention of HIV infection in women: Results of the HPTN 035 trial. Abstract 48LB (16th Conference on Retroviruses and Opportunistic Infections, 2009)
Buchbinder, S. P. et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomized, placebo-controlled, test-of-concept trial. Lancet 372, 1881–1893 (2008)
Check, E. Scientists rethink approach to HIV gels. Nature 446, 12 (2007)
Fauci, A. et al. HIV vaccine research: the way forward. Science 321, 530–532 (2008)
Quinn, T. C. & Overbaugh, J. HIV/AIDS in women: an expanding epidemic. Science 308, 1582–1583 (2005)
Haase, A. The slow infection caused by visna virus. Curr. Top. Microbiol. Immunol. 72, 101–156 (1975)
Miller, C. J. et al. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J. Virol. 63, 4277–4284 (1989)
Fiebig, E. W. et al. Dynamics of HIV viremia and seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 17, 1871–1879 (2003)
Miller, C. J. et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 79, 9217–9227 (2005)Comprehensive tissue analysis of the pathogenesis of transmission and early infection in the nonhuman primate model of vaginal transmission of HIV-1.
Hu, J., Gardner, M. B. & Miller, C. J. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74, 6087–6095 (2000)
Zhang, Z.-Q. et al. Sexual transmission and propagation of simian and human immunodeficiency viruses in two distinguishable populations of CD4+ T cells. Science 286, 1353–1357 (1999)First description of the CD4 T cell as the principal target in acute SIV and HIV-1 infections, and the surprising ostensibly resting phenotype of a majority of the CD4 T cells initially infected.
Reinhart, T. et al. Simian immunodeficiency virus burden in tissues and cellular compartments during clinical latency and AIDS. J. Infect. Dis. 176, 1198–1208 (1997)
Haase, A. T. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17, 625–656 (1999)
Miller, C. M. & Shattock, R. J. Target cells in vaginal HIV transmission. Microbes Infect. 5, 59–67 (2003)
Christopher, D. P. et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J. Infect. Dis. 189, 1785–1792 (2004)
Keele, B. F. et al. Low-dose rectal inoculation of rhesus macaques by SIV smE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206, 1117–1134 (2009)
Sodora, D. L., Gettie, A., Miller, C. J. & Marx, P. A. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res. Hum. Retroviruses 14 (Suppl. 1). S119–S123 (1998)
Weiler, A. M. et al. Genital ulcers facilitate rapid viral entry and dissemination following intravaginal inoculation with cell-associated simian immunodeficiency virus SIVmac239. J. Virol. 82, 4154–4158 (2008)
Miyake, A. et al. Rapid dissemination of a pathogenic simian/human immunodeficiency virus to systemic organs and active replication in lymphoid tissues following intrarectal infection. J. Gen. Virol. 87, 1311–1320 (2006)
Stahl-Hennig, C. et al. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science 285, 1261–1265 (1999)
Milush, J. M. et al. Rapid dissemination of SIV following oral inoculation. AIDS 18, 1–10 (2004)
Veazey, R. S. et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280, 427–431 (1998)
Mattapallil, J. et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434, 1093–1097 (2005)
Li, Q. et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434, 1148–1152 (2005)Tissue analysis that revealed the rapid kinetics of acute SIV infection after vaginal exposure, predominance of productive infection in resting memory CD4 T cells, and the loss of gut lamina propria CD4 T cells mediated mainly by apoptosis.
Clayton, F., Snow, G., Reka, S. & Kotler, D. P. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin. Exp. Immunol. 107, 288–292 (1997)
Mehandru, S. et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200, 761–770 (2004)
Brenchley, J. M. et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200, 749–759 (2004)
Guadalupe, M. et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77, 11708–11717 (2003)
Kotler, D. P., Gaetz, H. P., Lange, M., Klein, E. B. & Holt, P. R. Enteropathy associated with the acquired immunodeficiency syndrome. Ann. Intern. Med. 101, 421–428 (1984)
Heise, C., Miller, C. J., Lackner, A. & Dandekar, S. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J. Infect. Dis. 169, 1116–1120 (1994)
Li, Q. et al. Simian immunodeficiency virus-induced intestinal cell apoptosis is the underlying mechanism of the regenerative enteropathy of early infection. J. Infect. Dis. 197, 420–429 (2008)
Estes, J. D. et al. Premature induction of an immunosuppressive T regulatory response in acute SIV infection. J. Infect. Dis. 193, 703–712 (2006)
Reynolds, M. R. et al. The CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to SIV: too late and too little. J. Virol. 79, 9228–9235 (2005)Comprehensive tissue analysis after vaginal exposure that revealed the ‘too late and too little’ CD8 T-cell response to prevent massive gut CD4 T-cell depletion in acute SIV infection but a robust response in cervical vaginal tissues that could potentially prevent acquisition if elicited earlier by a vaccine.
Brenchley, J. M. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Med. 12, 1365–1371 (2006)
Estes, J. D. et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor β1-positive regulatory T cells and begins in early infection. J. Infect. Dis. 195, 551–561 (2007)
Estes, J. D., Haase, A. T. & Schacker, T. W. The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution of HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin. Immunol. 20, 181–186 (2008)
Wolinsky, S. et al. Selective transmission of human deficiency virus type 1 variants from mothers to infants. Science 255, 1134–1137 (1992)
Zhu, T. et al. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J. Virol. 70, 3098–3107 (1996)
Derdeyn, C. A. et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 303, 2019–2022 (2004)First description of the monophyletic nature of viruses transmitted heterosexually.
Keele, B. F. et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl Acad. Sci. USA 105, 7552–7557 (2008)Comprehensive sequence analysis showing that a single virus genotype initiates the vast majority of HIV-1 infections.
Salazar-Gonzalez, J. F. et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 206, 1273–1289 (2009)
Norvell, M. K., Benrubi, G. I. & Thompson, R. J. Investigation of microtrauma after sexual intercourse. J. Reprod. Med. 269, 269–271 (1984)
Pudney, J., Quayle, A. J. & Anderson, D. J. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol. Reprod. 73, 1253–1263 (2005)
O’Connor, D. M. A tissue basis for colposcopic findings. Obstet. Gynecol. Clin. North Am. 35, 565–582 (2008)
Li, Q. et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458, 1034–1038 (2009)Tissue analysis of vaginal transmission revealing local expansion of small, infected founder populations, in vivo evidence of mucosal epithelial signalling that elicited an inflammatory response to fuel local expansion, and the ability of glycerol monolaurate to prevent transmission potentially by blocking signalling at mucosal front lines.
Myer, L., Wright, T. C., Denny, L. & Kuhn, L. Nested case-control study of cervical mucosal lesions ectopy, and incident HIV infection among women in Cape Town, South Africa. Sex. Transm. Dis. 33, 683–687 (2006)
Miller, C. J., Alexander, N. J., Vogel, P., Anderson, J. & Marx, P. A. Mechanism of genital transmission of SIV: a hypothesis based on transmission studies and location of SIV in the genital tract of chronically infected female rhesus macaques. J. Med. Primatol. 21, 64–68 (1992)
Kell, P. D., Barton, S. E., Edmonds, D. K. & Boag, F. C. HIV infection in a patient with Meyer-Rokitansky-Kuster-Hauser syndrome. J. R. Soc. Med. 85, 706–707 (1992)
Padian, N. S. et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet 370, 251–261 (2007)
Berger, E. A., Murphy, P. M. & Farber, J. M. Chemokine receptors as HIV-1 coreceptors: Roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17, 657–700 (1999)
Arthos, J. et al. HIV-1 envelope binds to and signals through integrin α4β7, the gut mucosal homing receptor for peripheral T cells. Nature Immunol. 9, 301–309 (2008)
Kader, M. et al. α4β7hiCD4+ memory T cells harbor most TH -17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2, 439–449 (2009)
Zhang, Z.-Q. et al. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc. Natl Acad. Sci. USA 101, 5640–5645 (2004)
Schacker, T. S. et al. Productive infection of T cells in lymphoid tissues during primary and early human immunodeficiency virus infection. J. Infect. Dis. 183, 555–562 (2001)
Ma, Z., Lü, F. X., Torten, M. & Miller, C. J. The number and distribution of immune cells in the cervicovaginal mucosa remain constant throughout the menstrual cycle of rhesus macaques. Clin. Immunol. 100, 240–249 (2001)
Edwards, J. N. T. & Morris, H. B. Langerhans’ cells and lymphocyte subsets in the female genital tract. Br. J. Obstet. Gynaecol. 92, 974–982 (1985)
Wira, C. R., Fahey, J. V., Sentman, C. L., Pioli, P. A. & Shen, L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol. Rev. 206, 306–335 (2005)
Wira, C. R., Grant-Tschudy, K. S. & Crane-Godreau, M. A. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am. J. Reprod. Immunol. 53, 65–76 (2005)
Fahey, J. V. et al. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol. 1, 317–325 (2008)
Dieu-nosjean, M. et al. Macrophage inflammatory protein 3α is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J. Exp. Med. 192, 705–717 (2000)
Cremel, M. et al. Characterization of CCL20 secretion by human epithelial vaginal cells: involvement in Langerhans cell precursor attraction. J. Leukoc. Biol. 78, 158–166 (2005)
Abel, K., Rocke, D. M., Chohan, B., Fritts, L. & Miller, C. J. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J. Virol. 79, 12164–12172 (2005)
Gray, R. H. et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 357, 1149–1153 (2001)
Wawer, M. J. et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis. 191, 1403–1409 (2005)
Vendrame, D., Sourisseau, M., Perrin, V., Schwartz, O. & Mamano, F. Partial inhibition of human immunodeficiency virus replication by type I interferons: Impact of cell-to-cell viral transfer. J. Virol. 83, 10527–10537 (2009)
Rudnicka, D. et al. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J. Virol. 83, 6234–6246 (2009)
Wang, Y. et al. The toll-like receptor 7 (TLR7) agonist, imiquimod and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. J. Virol. 79, 14355–14370 (2005)
Glavin, S. R. & Cohen, M. S. The role of sexually transmitted diseases in HIV transmission. Nature Rev. Microbiol. 2, 33–42 (2004)
Kaul, R. et al. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. J. Reprod. Immunol. 77, 32–40 (2008)
Haaland, R. E. et al. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 5, 1–13 (2009)
Celum, C. et al. Effect of acyclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomized, double-blind, placebo-controlled trial. Lancet 371, 2109–2119 (2008)
Watson-Jones, D. et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N. Engl. J. Med. 358, 1560–1571 (2008)
Zhu, J. et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nature Med. 15, 886–893 (2009)Report of persistent target cells in healed HSV-2 ulcers after acyclovir treatment as the potential explanation for the failure of treatment to reduce HIV-1 acquisition.
Robertson, S. A. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 322, 43–52 (2005)
Robertson, S. A., Ingman, W. V., O’Leary, S., Sharkey, D. J. & Tremellen, K. P. Transforming growth factor β—a mediator of immune deviation in seminal plasma. J. Reprod. Immunol. 57, 109–128 (2002)
Berlier, W. et al. Seminal plasma promotes the attraction of Langerhans cells via the secretion of CCL20 by vaginal epithelial cells: involvement in the sexual transmission of HIV. Hum. Reprod. 21, 1135–1142 (2006)
Münch, J. et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 131, 1059–1071 (2007)
Li, Q. et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science 323, 1726–1729 (2009)Report of the tissue-compartment-specific nature of the cellular immune response that reveals the importance of the relative numbers of both immune effectors and infected targets in determining whether viral infection is cleared, and the extent of control if infection is not cleared.
Belyakov, I. M. et al. Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood 107, 3258–3264 (2006)
Genescà, M., Skinner, P. J., Bost, K. M., Lu, D. & Wang, Y. Protective attenuated lentivirus immunization induces SIV-specific T cells in the genital tract of rhesus monkeys. Mucosal Immunol. 1, 219–228 (2008)
Hansen, S. G. et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nature Med. 15, 293–299 (2009)Report of impressive protection against rectal transmission associated with effector memory T-cell responses elicited by a rhesus CMV vaccine.
Lifson, J. D. et al. Containment of simian immunodeficiency virus infection: Cellular immune responses and protection from rechallenge following transient postinoculation antiretroviral treatment. J. Virol. 74, 2584–2593 (2000)
Veazey, R. S. et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature 438, 99–102 (2005)
Lederman, M. M. et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science 306, 485–487 (2004)
Cranage, M. et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 5, 1238–1250 (2008)
Zhu, T. et al. Persistence of extraordinarily low levels of genetically homogeneous human immunodeficiency virus type 1 in exposed seronegative individuals. J. Virol. 77, 6108–6116 (2003)
McChesney, M. B. et al. Occult systemic infection and persistent simian immunodeficiency virus (SIV)-specific CD4+-T-cell proliferative responses in rhesus macaques that were transiently viremic after intravaginal inoculation of SIV. J. Virol. 72, 10029–10035 (1998)
Ma, Z.-M., Abel, K., Rourke, T., Wang, Y. & Miller, C. J. A period of transient viremia and occult infection precedes persistent viremia and antiviral immune responses during multiple low-dose intravaginal simian immunodeficiency virus inoculations. J. Virol. 78, 14048–14052 (2004)
Trivedi, P. et al. Intrarectal transmission of simian immunodeficiency virus in rhesus macaques: selective amplification and host responses to transient or persistent viremia. J. Virol. 70, 6876–6883 (1996)
Li, Q. et al. Microarray analysis of lymphatic tissue reveals stage-specific, gene expression signatures in HIV-1 infection. J. Immunol. 183, 1975–1982 (2009)
Virgin, H. W. & Walker, B. D. Immunology and the elusive AIDS vaccine. Nature (in the press)
Acknowledgements
I thank J. V. Carlis, R. P. Johnson, Q. Li, J. D. Lifson, D. Masopust, S. Pambuccian, P. J. Southern, J. Estes, D. Douek, H. W. Virgin and B. D. Walker for discussions. Errors of commission are mine as are errors of omission, with apologies to the authors of work that I could not cite because of space limitations and exclusive focus on tissue analyses. I thank C. O’Neill and T. Leonard for help with the manuscript and figures. Work from my laboratory cited in the review was supported by grants from the National Institutes of Health (AI 38565, AI 48484, AI 71976) and the International AIDS Vaccine Initiative.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Haase, A. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464, 217–223 (2010). https://doi.org/10.1038/nature08757
Issue Date:
DOI: https://doi.org/10.1038/nature08757
This article is cited by
-
Immune activation of vaginal human Langerhans cells increases susceptibility to HIV-1 infection
Scientific Reports (2023)
-
HIV-1 subverts the complement system in semen to enhance viral transmission
Mucosal Immunology (2021)
-
New GMP manufacturing processes to obtain thermostable HIV-1 gp41 virosomes under solid forms for various mucosal vaccination routes
npj Vaccines (2020)
-
The epidemiology of HIV and other sexually transmitted infections in African, Caribbean and Black men in Toronto, Canada
BMC Infectious Diseases (2019)
-
Interferon-β induced in female genital epithelium by HIV-1 glycoprotein 120 via Toll-like-receptor 2 pathway acts to protect the mucosal barrier
Cellular & Molecular Immunology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.