Abstract
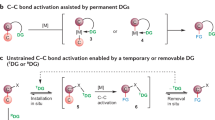
The cleavage of C–H and C–C bonds by transition metal centres is of fundamental interest and plays an important role in the synthesis of complex organic molecules from petroleum feedstocks1,2,3,4,5,6. But while there are many examples for the oxidative addition of C–H bonds to a metal centre, transformations that feature oxidative addition of C–C bonds are rare. The paucity of transformations that involve the cleavage of C–C rather than C–H bonds is usually attributed to kinetic factors arising from the greater steric hindrance and the directional nature of the sp n hybrids that form the C–C bond, and to thermodynamic factors arising from the fact that M–C bonds are weaker than M–H bonds2,3,4,5. Not surprisingly, therefore, most examples of C–C bond cleavage either avoid the kinetic limitations by using metal compounds in which the C–C bond is held in close proximity to the metal centre, or avoid the thermodynamic limitations by using organic substrates in which the cleavage is accompanied by either a relief of strain energy or the formation of an aromatic system2,3,4,5. Here, we show that a tungsten centre can be used to cleave a strong C–C bond that is a component of an unstrained 6-membered aromatic ring. The cleavage is enabled by the formation of an unusual chelating di(isocyanide) ligand, which suggests that other metal centres with suitable ancillary ligands could also accomplish the cleavage of strong C–C bonds of aromatic substrates and thereby provide new ways of functionalizing such molecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jones, W. D. in Comprehensive Organometallic Chemistry III, Vol. 1, Ch. 1.25 (eds Crabtree, R. H. & Mingos, D. M. P.) (Elsevier, 2006)
Jones, W. D. The fall of the C–C bond. Nature 364, 676–677 (1993)
Jun, C. H. Transition metal-catalyzed carbon-carbon bond activation. Chem. Soc. Rev. 33, 610–618 (2004)
van der Boom, M. E. & Milstein, D. Cyclometalated phosphine-based pincer complexes: Mechanistic insight in catalysis, coordination, and bond activation. Chem. Rev. 103, 1759–1792 (2003)
Crabtree, R. H. The organometallic chemistry of alkanes. Chem. Rev. 85, 245–269 (1985)
Rybtchinski, B. & Milstein, D. Metal insertion into C–C bonds in solution. Angew. Chem. Int. Edn Engl. 38, 870–883 (1999)
Zhu, G., Tanski, J. M., Churchill, D. G., Janak, K. E., & Parkin, G. The reactivity of Mo(PMe3)6 towards heterocyclic nitrogen compounds: transformations relevant to hydrodenitrogenation. J. Am. Chem. Soc. 124, 13658–13659 (2002)
Zhu, G., Pang, K. & Parkin, G. New modes for coordination of aromatic heterocyclic nitrogen compounds to molybdenum: catalytic hydrogenation of quinoline, isoquinoline, and quinoxaline by Mo(PMe3)4H4 . J. Am. Chem. Soc. 130, 1564–1565 (2008)
Buccella, D. & Parkin, G. p-tert-butylcalix[4] arene complexes of molybdenum and tungsten: reactivity of the calixarene methylene C-H bond and the facile migration of the metal around the phenolic rim of the calixarene. J. Am. Chem. Soc. 128, 16358–16364 (2006)
Cyranski, M. K. Energetic aspects of cyclic pi-electron delocalization: evaluation of the methods of estimating aromatic stabilization energies. Chem. Rev. 105, 3773–3811 (2005)
Kleckley, T. S., Bennett, J. L., Wolczanski, P. T. & Lobkovsky, E. B. Pyridine C = N bond cleavage mediated by (silox)3Nb(silox = tBu3SiO). J. Am. Chem. Soc. 119, 247–248 (1997)
Bonanno, J. B., Veige, A. S., Wolczanski, P. T. & Lobkovsky, E. B. Amide derivatives of tantalum and a niobium-promoted ring opening of 3,5-lutidine. Inorg. Chim. Acta 345, 173–184 (2003)
Gray, S. D., Weller, K. J., Bruck, M. A., Briggs, P. M. & Wigley, D. E. Carbon-nitrogen bond-cleavage in an η2(N, C)-pyridine complex-induced by intramolecular metal-to-ligand alkyl migration - models for hydrodenitrogenation catalysis. J. Am. Chem. Soc. 117, 10678–10693 (1995)
Weller, K. J., Filippov, I., Briggs, P. M. & Wigley, D. E. Pyridine degradation intermediates as models for hydrodenitrogenation catalysis: preparation and properties of a metallapyridine complex. Organometallics 17, 322–329 (1998)
Bailey, B. C., Fan, H., Huffman, J. C., Baik, M. H. & Mindiola, D. J. Room temperature ring-opening metathesis of pyridines by a transient Ti≡C linkage. J. Am. Chem. Soc. 128, 6798–6799 (2006)
Ito, Y., Ohnishi, A., Ohsaki, H. & Murakami, M. A preparative method for ortho-diisocyanoarenes. Synthesis 714–715 (1988)
Wagner, N. L., Laib, F. E. & Bennett, D. W. Conformational isomerism in (p-RC6H4NC)2W(dppe)2: substantial structural changes resulting from subtle differences in the pi-acidity of p-RC6H4NC. J. Am. Chem. Soc. 122, 10856–10867 (2000)
Kuznetsov, M. L. Theoretical studies of transition metal complexes with nitriles and isocyanides. Russ. Chem. Rev. 71, 265–282 (2002)
Strauch, H. C. et al. Reactions of (butadiene)tantalocene cation with alkyl isocyanides. Organometallics 18, 3802–3812 (1999)
Brennessel, W. W. & Ellis, J. E. [Fe(CNXyl)4]2-: an isolable and structurally characterized homoleptic isocyanidemetalate dianion. Angew. Chem. Int. Edn Engl. 46, 598–600 (2007)
Hahn, F. E. The coordination chemistry of multidentate isocyanide ligands. Angew. Chem. Int. Edn Engl. 32, 650–665 (1993)
Espinet, P., Soulantica, K., Charmant, J. P. H. & Orpen, A. G. 1,2-Phenylene diisocyanide for metallotriangles. Chem. Commun. 915–916 (2000)
Hahn, F. E., Tamm, M., Imhof, L. & Lugger, T. Preparation and crystal structures of a bidentate isocyanide and its tetracarbonylchromium complex. J. Organomet. Chem. 526, 149–155 (1996)
Lappert, M. F. Contributions to the chemistry of carbene metal chemistry. J. Organomet. Chem. 690, 5467–5473 (2005)
Wilker, C. N., Hoffmann, R. & Eisenstein, O. Coupling methylenes, methynes and other π-systems on one or two metal centers. Nouv. J. Chim. 7, 535–544 (1983)
Hartwig, J. F., Bergman, R. G. & Andersen, R. A. Structure, synthesis, and chemistry of (PMe3)4Ru(η2-benzyne). Reactions with arenes, alkenes, and heteroatom-containing organic-compounds. Synthesis and structure of a monomeric hydroxide complex. J. Am. Chem. Soc. 113, 3404–3418 (1991)
Pombeiro, A. J. L., da Silva, M. & Michelin, R. A. Aminocarbyne complexes derived from isocyanides activated towards electrophilic addition. Coord. Chem. Rev. 218, 43–74 (2001)
Carnahan, E. M., Protasiewicz, J. D. & Lippard, S. J. 15 years of reductive coupling: what have we learned? Acc. Chem. Res. 26, 90–97 (1993)
Ito, Y. et al. Aromatizing oligomerization of 1,2-di-isocyanoarene to quinoxaline oligomers. J. Chem. Soc. Chem. Commun. 403–405 (1990)
Clauss, A. D., Shapley, J. R., Wilker, C. N. & Hoffmann, R. Alkyne scission on a trimetallic framework: experimental evidence and theoretical analysis. Organometallics 3, 619 (1984)
Acknowledgements
We thank the US Department of Energy, Office of Basic Energy Sciences (DE-FG02-93ER14339) for supporting this research. We thank The National Science Foundation (CHE-0619638) for the acquisition of an X-ray diffractometer. We thank W. Sattler for discussions.
Author Contributions G.P. supervised the project. A.S. synthesized and characterized the compounds. G.P. and A.S. analysed the data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors declare no competing financial interests. X-ray crystallographic coordinates have been deposited at the Cambridge Crystallographic Database (CCDC #734113–734121).
Supplementary information
Supplementary Information
This file contains Supplementary Experiments, Supplementary Figures S1- S9 with Legends, Supplementary Table 1 and Supplementary References. (PDF 2891 kb)
Rights and permissions
About this article
Cite this article
Sattler, A., Parkin, G. Cleaving carbon–carbon bonds by inserting tungsten into unstrained aromatic rings. Nature 463, 523–526 (2010). https://doi.org/10.1038/nature08730
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08730
This article is cited by
-
Tunable molecular editing of indoles with fluoroalkyl carbenes
Nature Chemistry (2024)
-
Cleavage of non-polar C(sp2)‒C(sp2) bonds in cycloparaphenylenes via electric field-catalyzed electrophilic aromatic substitution
Nature Communications (2023)
-
Decarboxylative oxidation-enabled consecutive C-C bond cleavage
Nature Communications (2022)
-
Cleaving arene rings for acyclic alkenylnitrile synthesis
Nature (2021)
-
Hydrodenitrogenation of pyridines and quinolines at a multinuclear titanium hydride framework
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.