Abstract
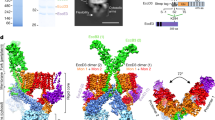
Type IV secretion systems are secretion nanomachines spanning the two membranes of Gram-negative bacteria. Three proteins, VirB7, VirB9 and VirB10, assemble into a 1.05 megadalton (MDa) core spanning the inner and outer membranes. This core consists of 14 copies of each of the proteins and forms two layers, the I and O layers, inserting in the inner and outer membrane, respectively. Here we present the crystal structure of a ∼0.6 MDa outer-membrane complex containing the entire O layer. This structure is the largest determined for an outer-membrane channel and is unprecedented in being composed of three proteins. Unexpectedly, this structure identifies VirB10 as the outer-membrane channel with a unique hydrophobic double-helical transmembrane region. This structure establishes VirB10 as the only known protein crossing both membranes of Gram-negative bacteria. Comparison of the cryo-electron microscopy (cryo-EM) and crystallographic structures points to conformational changes regulating channel opening and closing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fronzes, R., Christie, P. J. & Waksman, G. The structural biology of type IV secretion systems. Nature Rev. Microbiol. 7, 703–714 (2009)
Christie, P. J., Atmakuri, K., Krishnamoorthy, V., Jakubowski, S. & Cascales, E. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59, 451–485 (2005)
Schroder, G. & Lanka, E. The mating pair formation system of conjugative plasmids – a versatile secretion machinery for transfer of proteins and DNA. Plasmid 54, 1–25 (2005)
Backert, S. & Selbach, M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell. Microbiol. 10, 1573–1581 (2008)
Ninio, S. & Roy, C. R. Effector proteins translocated by Legionella pneumophila: strength in numbers. Trends Microbiol. 15, 372–380 (2007)
McCullen, C. A. & Binns, A. N. Agrobacterium tumefaciens and plant cell interactions and activities required for interkingdom macromolecular transfer. Annu. Rev. Cell Dev. Biol. 22, 101–127 (2006)
Burns, D. L. Type IV transporters of pathogenic bacteria. Curr. Opin. Microbiol. 6, 29–34 (2003)
Thomas, C. M. & Nielsen, K. M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nature Rev. Microbiol. 3, 711–721 (2005)
Fronzes, R., Remaut, H. & Waksman, G. Architectures and biogenesis of non-flagellar protein appendages in Gram-negative bacteria. EMBO J. 27, 2271–2280 (2008)
Gomis-Ruth, F. X. & Coll, M. Cut and move: protein machinery for DNA processing in bacterial conjugation. Curr. Opin. Struct. Biol. 16, 744–752 (2006)
Bayliss, R. et al. NMR structure of a complex between the VirB9/VirB7 interaction domains of the pKM101 type IV secretion system. Proc. Natl Acad. Sci. USA 104, 1673–1678 (2007)
Fronzes, R. et al. Structure of a type IV secretion system core complex. Science 323, 266–268 (2009)
Jakubowski, S. J. et al. Agrobacterium VirB10 domain requirements for type IV secretion and T pilus biogenesis. Mol. Microbiol. 71, 779–794 (2009)
Terradot, L. et al. Structures of two core subunits of the bacterial type IV secretion system, VirB8 from Brucella suis and ComB10 from Helicobacter pylori . Proc. Natl Acad. Sci. USA 102, 4596–4601 (2005)
Dong, C. et al. Wza the translocon for E. coli capsular polysaccharides defines a new class of membrane protein. Nature 444, 226–229 (2006)
Meng, G., Fronzes, R., Chandran, V., Remaut, H. & Waksman, G. Protein oligomerization in the bacterial outer membrane. Mol. Membr. Biol. 26, 136–145 (2009)
Koronakis, V., Sharff, A., Koronakis, E., Luisi, B. & Hughes, C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405, 914–919 (2000)
Cascales, E. & Christie, P. J. Agrobacterium VirB10, an ATP energy sensor required for type IV secretion. Proc. Natl Acad. Sci. USA 101, 17228–17233 (2004)
Atmakuri, K., Cascales, E. & Christie, P. J. Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol. Microbiol. 54, 1199–1211 (2004)
Llosa, M., Zunzunegui, S. & de la Cruz, F. Conjugative coupling proteins interact with cognate and heterologous VirB10-like proteins while exhibiting specificity for cognate relaxosomes. Proc. Natl Acad. Sci. USA 100, 10465–10470 (2003)
Cascales, E. & Christie, P. J. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 304, 1170–1173 (2004)
Sharff, A. J., Koronakis, E., Luisi, B. & Koronakis, V. Oxidation of selenomethionine: some MADness in the method! Acta Crystallogr. D 56, 785–788 (2000)
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Cryst. 26, 795–800 (1993)
Navaza, J. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (1994)
Jones, T. A. in Molecular Replacement 91–95 (CCP4 Proceedings, 1992) 〈http://epubs.cclrc.ac.uk/bitstream/948/DL-SCI-R33.pdf〉.
Kjeldgaard, M. & Jones, T. A. in From First Map to Final Model 59–66 (CCP4 Proceedings, 1994) 〈http://epubs.cclrc.ac.uk/bitstream/950/DL-SCI-R35.pdf〉.
Kleywegt, G. & Jones, T. Software for handling macromolecular envelopes. Acta Crystallogr. D 55, 941–944 (1999)
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
McCoy, A. et al. Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007)
Murshudov, G., Vagin, A. & Dodson, E. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997)
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D 58, 1948–1954 (2002)
Afonine, P. V., Grosse-Kunstleve, R. W. & Adams, P. D. The Phenix refinement framework. CCP4 Newsl. 42, contribution 8 (2005) 〈http://www.ccp4.ac.uk/newsletters/newsletter42.pdf〉.
Acknowledgements
This work was funded by Wellcome Trust grant 082227 to G.W. We thank A. Thompson and the staff of beamline PROXIMA 1 at Soleil, the staff of beamline ID14.4 at the European Synchrotron Radiation Facility, and H. Saibil, E. Orlova and P. Christie for comments on the manuscript. We thank A. Kumar for help in implementing the immunofluorescence experiments.
Author Contributions V.C. produced the complex, optimized crystals, and built, refined and analysed the structure. R.F. designed the purification protocol, produced the complex, grew the first crystals, optimized crystals and analysed the structure. S.D. and J.N. solved the structure by molecular replacement and provided the electron density map. N.C. collected crystallographic data. G.W. supervised the work, analysed the structure and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion, Supplementary Table 1, Supplementary Figures 1-10 with Legends and Supplementary References. (PDF 13100 kb)
Rights and permissions
About this article
Cite this article
Chandran, V., Fronzes, R., Duquerroy, S. et al. Structure of the outer membrane complex of a type IV secretion system . Nature 462, 1011–1015 (2009). https://doi.org/10.1038/nature08588
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08588
This article is cited by
-
Structural and functional diversity of type IV secretion systems
Nature Reviews Microbiology (2024)
-
Structure of a type IV secretion system core complex encoded by multi-drug resistance F plasmids
Nature Communications (2022)
-
Architecture of the outer-membrane core complex from a conjugative type IV secretion system
Nature Communications (2021)
-
Identification and evaluation of UL36 protein from Dermacentor silvarum salivary gland and its interaction with Anaplasma ovis VirB10
Parasites & Vectors (2020)
-
Architecture of type VI secretion system membrane core complex
Cell Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.