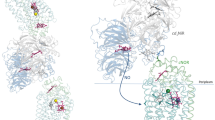
Abstract
Recent earth science studies have pointed out that massive acceleration of the global nitrogen cycle by anthropogenic addition of bio-available nitrogen has led to a host of environmental problems1. Nitrous oxide (N2O) is a greenhouse gas that is an intermediate during the biological process known as denitrification2. Copper-containing nitrite reductase (CuNIR) is a key enzyme in the process; it produces a precursor for N2O by catalysing the one-electron reduction of nitrite ( ) to nitric oxide (NO)3. The reduction step is performed by an efficient electron-transfer reaction with a redox-partner protein4,5,6. However, details of the mechanism during the electron-transfer reaction are still unknown. Here we show the high-resolution crystal structure of the electron-transfer complex for CuNIR with its cognate cytochrome c as the electron donor. The hydrophobic electron-transfer path is formed at the docking interface by desolvation owing to close contact between the two proteins. Structural analysis of the interface highlights an essential role for the loop region with a hydrophobic patch for protein–protein recognition; it also shows how interface construction allows the variation in atomic components to achieve diverse biological electron transfers.
) to nitric oxide (NO)3. The reduction step is performed by an efficient electron-transfer reaction with a redox-partner protein4,5,6. However, details of the mechanism during the electron-transfer reaction are still unknown. Here we show the high-resolution crystal structure of the electron-transfer complex for CuNIR with its cognate cytochrome c as the electron donor. The hydrophobic electron-transfer path is formed at the docking interface by desolvation owing to close contact between the two proteins. Structural analysis of the interface highlights an essential role for the loop region with a hydrophobic patch for protein–protein recognition; it also shows how interface construction allows the variation in atomic components to achieve diverse biological electron transfers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gruber, N. & Galloway, J. N. An earth-system perspective of the global nitrogen cycle. Nature 451, 293–296 (2008)
Firestone, M. K., Firestone, R. B. & Tiedje, J. M. Nitrous oxide from soil denitrification: factors controlling its biological production. Science 208, 749–751 (1980)
Zumft, W. G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61, 533–616 (1997)
Kataoka, K. et al. Structure-based engineering of Alacaligenes xylosoxidans copper-containing nitrite reductase enhances intermolecular electron transfer reaction with pseudoazurin. J. Biol. Chem. 279, 53374–53378 (2004)
Murphy, L. M., Dodd, F. E., Yousafzai, F. K., Eady, R. R. & Hasnain, S. S. Electron donation between copper containing nitrite reductases and cupredoxins: the nature of protein–protein interaction in complex formation. J. Mol. Biol. 315, 859–871 (2002)
Kukimoto, M. et al. Site-directed mutagenesis of azurin from Pseudomonas aeruginosa enhances the formation of an electron-transfer complex with a copper-containing nitrite reductase from Alcaligenes faecalis S-6. FEBS Lett. 394, 87–90 (1996)
Coyne, M. S., Arunakumari, A., Averill, B. A. & Tiedje, J. M. Immunological identification and distribution of dissimilatory heme cd 1 and nonheme copper nitrite reductases in denitrifying bacteria. Appl. Environ. Microbiol. 55, 2924–2931 (1989)
Vijgenboom, E., Busch, J. E. & Canters, G. W. In vivo studies disprove an obligatory role of azurin in denitrification in Pseudomonas aeruginosa and show that azu expression is under control of RpoS and ANR. Microbiology 143, 2853–2863 (1997)
Ferguson, S. J. & Richardson, D. J. in Respiration in Archaea and Bacteria, Vol. 2 (ed. Zannoni, D.) 169–206 (Springer, 2004)
Godden, J. W. et al. The 2.3 Angstrom X-ray structure of nitrite reductase from Achromobacter cycloclastes . Science 253, 438–442 (1991)
Suzuki, S., Kataoka, K. & Yamaguchi, K. Metal coordination and mechanism of multi-copper nitrite reductase. Acc. Chem. Res. 33, 728–735 (2000)
Dodd, F. E. et al. Evidence for two distinct azurins in Alcaligenes xylosoxidans (NCIMB 11015): potential electron donors to nitrite reductase. Biochemistry 34, 10180–10186 (1995)
Deligeer, K., Yamaguchi, K. & Suzuki, S. Spectroscopic and electrochemical properties of cytochrome c 551 from Alcaligenes xylosoxidans GIFU 1051. Bull. Chem. Soc. Jpn 73, 1839–1840 (2000)
Yabuuchi, E., Kawamura, Y., Kosako, Y. & Ezaki, T. Emendation of genus Achromobacter and Achromobacter xylosoxidans (Yabuuchi and Yano) and proposal of Achromobacter ruhlandii (Packer and Vishniac) comb. nov., Achromobacter piechaudii (Kiredjian et al.) comb. nov., and Achromobacter xylosoxidans subsp. denitrificans (Rüger and Tan) comb. nov. Microbiol. Immunol. 42, 429–438 (1998)
Hasegawa, N., Arai, H. & Igarashi, Y. Two c-type cytochromes, NirM and NirC, encoded in the nir gene cluster of Pseudomonas aeruginosa act as electron donors for nitrite reductase. Biochem. Biophys. Res. Commun. 288, 1223–1230 (2001)
Bueno, E., Bedmar, E. J., Richardson, D. J. & Delgado, M. J. Role of Bradyrhizobium japonicum cytochrome c 550 in nitrite and nitrate respiration. FEMS Microbiol. Lett. 279, 188–194 (2008)
Glockner, A. B., Jüngst, A. & Zumft, W. G. Copper-containing nitrite reductase from Pseudomonas aureofaciens is functional in a mutationally cytochrome cd 1-free background (NirS-) of Pseudomonas stutzeri . Arch. Microbiol. 160, 18–26 (1993)
Miyashita, O., Onuchic, J. N. & Okamura, M. Y. Continuum electrostatic model for the binding of cytochrome c 2 to the photosynthetic reaction center from Rhodobacter sphaeroides . Biochemistry 42, 11651–11660 (2003)
Axelrod, H. L. et al. X-ray structure determination of the cytochrome c 2: reaction center electron transfer complex from Rhodobacter sphaeroides . J. Mol. Biol. 319, 501–515 (2002)
Pelletier, H. & Kraut, J. Crystal structure of a complex between electron transfer partners, cytochrome c peroxidase and cytochrome c . Science 258, 1748–1755 (1992)
Solmaz, S. R. N. & Hunte, C. Structure of complex III with bound cytochrome c in reduced state and definition of a minimal core interface for electron transfer. J. Biol. Chem. 283, 17542–17549 (2008)
Boulanger, M. J. & Murphy, M. E. P. Crystal structure of the soluble domain of the major anaerobically induced outer membrane protein (AniA) from pathogenic Neisseria: a new class of copper-containing nitrite reductases. J. Mol. Biol. 315, 1111–1127 (2002)
Page, C. C., Moser, C. C., Chen, X. & Dutton, P. L. Natural engineering principles of electron tunnelling in biological oxidation-reduction. Nature 402, 47–52 (1999)
Onuchic, J. N., Beratan, D. N., Winkler, J. R. & Gray, H. B. Pathway analysis of protein electron-transfer reactions. Annu. Rev. Biophys. Biomol. Struct. 21, 349–377 (1992)
Vlasie, M. D., Fernández-Busnadiego, R., Prudêncio, M. & Ubbink, M. Conformation of pseudoazurin in the 152 kDa electron transfer complex with nitrite reductase determined by paramagnetic NMR. J. Mol. Biol. 375, 1405–1415 (2008)
Williams, P. A. et al. Pseudospecific docking surfaces on electron transfer proteins as illustrated by pseudoazurin, cytochrome c 550 and cytochrome cd 1 nitrite reductase. Nature Struct. Biol. 2, 975–982 (1995)
Canters, G. W. et al. The effect of pH and ligand exchange on the redox properties of blue copper proteins. Faraday Discuss. 116, 205–220 (2000)
Zhu, Z. et al. Molecular basis for interprotein complex-dependent effects on the redox properties of amicyanin. Biochemistry 37, 17128–17136 (1998)
Bertini, I., Cavallaro, G. & Rosato, A. Cytochrome c: occurrence and functions. Chem. Rev. 106, 90–115 (2006)
Enright, A. J., Iliopoulos, I., Kyrpides, N. C. & Ouzounis, C. A. Protein interaction maps for complete genomes based on gene fusion events. Nature 402, 86–90 (1999)
Iwasaki, H. et al. Cytochrome c′ isolated from Achromobacter xylosoxidans GIFU 1055. Plant Cell Physiol. 27, 733–736 (1986)
Masuko, M., Iwasaki, H., Sakurai, T., Suzuki, S. & Nakahara, A. Characterization of nitrite reductase from a denitrifier, Alcaligenes sp. NCIB 11015. A novel copper protein. J. Biochem. 96, 447–454 (1984)
Suzuki, H. & Iwasaki, H. Studies on denitrification VI. Preparations and properties of crystalline blue protein and cryptocytochrome c, and role of copper in denitrifying enzyme from a denitrifying bacterium. J. Biochem. 52, 193–199 (1962)
Suzuki, S. et al. Spectroscopic characterization and intramolecular electron transfer processes of native and type 2 Cu-depleted nitrite reductases. J. Biol. Inorg. Chem. 2, 265–274 (1997)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Ellis, M. J., Dodd, F. E., Sawers, G., Eady, R. R. & Hasnain, S. S. Atomic resolution structures of native copper nitrite reductase from Alcaligenes xylosoxidans and the active site mutant Asp92Glu. J. Mol. Biol. 328, 429–438 (2003)
Vagin, A. & Teplyakov, A. MOLREP: an automated program for molecular replacement. J. Appl. Cryst. 30, 1022–1025 (1997)
Brünger, A. T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
McRee, D. E. XtalView/Xfit—A versatile program for manipulating atomic coordinates and electron density. J. Struct. Biol. 125, 156–165 (1999)
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997)
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 26, 283–291 (1993)
Lovell, S. C. et al. Structure validation by Cα geometry: φ, ψ and Cβ deviation. Proteins 50, 437–450 (2003)
Kataoka, K., Furusawa, H., Takagi, K., Yamaguchi, K. & Suzuki, S. Functional analysis of conserved aspartate and histidine residues located around the type 2 copper site of copper-containing nitrite reductase. J. Biochem. 127, 345–350 (2000)
Acknowledgements
We thank A. Nakagawa, M. Suzuki, M. Yoshimura and E. Yamashita (beamline 44XU at SPring-8) for their support in the collection of X-ray data (proposal numbers 2007A6918 and 2007B6918, to M.N.). This work was supported in part by Grants-in-Aids for Scientific Research 20350078 (to S.S.) and Encouragement of Young Scientists 20750137 (to M.N.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a Grant for Basic Science Research Projects from the Sumitomo Foundation (to M.N.).
Author Contributions M.N., H.K. and S.S. conceived and designed the project; M.N., H.K., T.N. and K.Y. purified; M.N. and H.K. crystallized; M.N., H.K., K.K. and T.I. conducted experimental works including data collection and structure analysis; M.N. and H.K. performed stopped-flow kinetics and analysed; M.N. and S.S. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-3, Supplementary Figures 1-7 with Legends and a Supplementary Reference. (PDF 8954 kb)
Rights and permissions
About this article
Cite this article
Nojiri, M., Koteishi, H., Nakagami, T. et al. Structural basis of inter-protein electron transfer for nitrite reduction in denitrification . Nature 462, 117–120 (2009). https://doi.org/10.1038/nature08507
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08507
This article is cited by
-
Cloning, purification and characterization of novel Cu-containing nitrite reductase from the Bacillus firmus GY-49
World Journal of Microbiology and Biotechnology (2018)
-
Highly diverse nirK genes comprise two major clades that harbour ammonium-producing denitrifiers
BMC Genomics (2016)
-
Aerobic denitrification: A review of important advances of the last 30 years
Biotechnology and Bioprocess Engineering (2015)
-
Impact of residues remote from the catalytic centre on enzyme catalysis of copper nitrite reductase
Nature Communications (2014)
-
Structures of protein–protein complexes involved in electron transfer
Nature (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.