Abstract
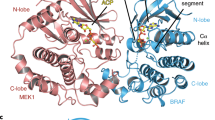
The ERK (extracellular signal-regulated kinase) pathway is an evolutionarily conserved signal transduction module that controls cellular growth, differentiation and survival1. Activation of receptor tyrosine kinases (RTKs) by the binding of growth factors initiates GTP loading of RAS, which triggers the initial steps in the activation of the ERK pathway by modulating RAF family kinase function. Once activated, RAF participates in a sequential cascade of phosphorylation events that activate MEK, and in turn ERK. Unbridled signalling through the ERK pathway caused by activating mutations in RTKs, RAS or RAF has been linked to several human cancers2. Of note, one member of the RAF family, BRAF, is the most frequently mutated oncogene in the kinase superfamily3. Not surprisingly, there has been a colossal effort to understand the underlying regulation of this family of kinases. In particular, the process by which the RAF kinase domain becomes activated towards its substrate MEK remains of topical interest. Here, using Drosophila Schneider S2 cells, we demonstrate that RAF catalytic function is regulated in response to a specific mode of dimerization of its kinase domain, which we term the side-to-side dimer. Moreover, we find that the RAF-related pseudo-kinase KSR (kinase suppressor of Ras) also participates in forming side-to-side heterodimers with RAF and can thereby trigger RAF activation. This mechanism provides an elegant explanation for the longstanding conundrum about RAF catalytic activation, and also provides an explanation for the capacity of KSR, despite lacking catalytic function, to directly mediate RAF activation. We also show that RAF side-to-side dimer formation is essential for aberrant signalling by oncogenic BRAF mutants, and identify an oncogenic mutation that acts specifically by promoting side-to-side dimerization. Together, our data identify the side-to-side dimer interface of RAF as a potential therapeutic target for intervention in BRAF-dependent tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Wellbrock, C., Karasarides, M. & Marais, R. The RAF proteins take centre stage. Nature Rev. Mol. Cell Biol. 5, 875–885 (2004)
Roberts, P. J. & Der, C. J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26, 3291–3310 (2007)
Greenman, C. et al. Patterns of somatic mutation in human cancer genomes. Nature 446, 153–158 (2007)
Manning, G., Whyte, D. B., Martinez, R., Hunter, T. & Sudarsanam, S. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002)
Boudeau, J., Miranda-Saavedra, D., Barton, G. J. & Alessi, D. R. Emerging roles of pseudokinases. Trends Cell Biol. 16, 443–452 (2006)
Kolch, W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nature Rev. Mol. Cell Biol. 6, 827–837 (2005)
Douziech, M., Sahmi, M., Laberge, G. & Therrien, M. A. KSR/CNK complex mediated by HYP, a novel SAM domain-containing protein, regulates RAS-dependent RAF activation in Drosophila . Genes Dev. 20, 807–819 (2006)
Roy, F., Laberge, G., Douziech, M., Ferland-McCollough, D. & Therrien, M. KSR is a scaffold required for activation of the ERK/MAPK module. Genes Dev. 16, 427–438 (2002)
Roignant, J. Y., Hamel, S., Janody, F. & Treisman, J. E. The novel SAM domain protein Aveugle is required for Raf activation in the Drosophila EGF receptor signaling pathway. Genes Dev. 20, 795–806 (2006)
Ritt, D. A. et al. CK2 is a component of the KSR1 scaffold complex that contributes to Raf kinase activation. Curr. Biol. 17, 179–184 (2007)
Wan, P. T. et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116, 855–867 (2004)
King, A. J. et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res. 66, 11100–11105 (2006)
Tsai, J. et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc. Natl Acad. Sci. USA 105, 3041–3046 (2008)
Hansen, J. D. et al. Potent and selective pyrazole-based inhibitors of B-Raf kinase. Bioorg. Med. Chem. Lett. 18, 4692–4695 (2008)
Huse, M. & Kuriyan, J. The conformational plasticity of protein kinases. Cell 109, 275–282 (2002)
Dar, A. C., Dever, T. E. & Sicheri, F. Higher-order substrate recognition of eIF2α by the RNA-dependent protein kinase PKR. Cell 122, 887–900 (2005)
Zhang, X., Gureasko, J., Shen, K., Cole, P. A. & Kuriyan, J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 125, 1137–1149 (2006)
Muthuswamy, S. K., Gilman, M. & Brugge, J. S. Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol. Cell. Biol. 19, 6845–6857 (1999)
Weber, C. K., Slupsky, J. R., Kalmes, H. A. & Rapp, U. R. Active Ras induces heterodimerization of cRaf and BRaf. Cancer Res. 61, 3595–3598 (2001)
Garnett, M. J., Rana, S., Paterson, H., Barford, D. & Marais, R. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol. Cell 20, 963–969 (2005)
Rushworth, L. K., Hindley, A. D., O’Neill, E. & Kolch, W. Regulation and role of Raf-1/B-Raf heterodimerization. Mol. Cell. Biol. 26, 2262–2272 (2006)
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002)
Halilovic, E. & Solit, D. B. Therapeutic strategies for inhibiting oncogenic BRAF signaling. Curr. Opin. Pharmacol. 8, 419–426 (2008)
Schwede, T., Kopp, J., Guex, N. & Peitsch, M. C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 31, 3381–3385 (2003)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Acknowledgements
We thank members of the F.S. and M.T. laboratories for advice and discussions. We are also grateful to R. Lambert and IRIC genomic platform for conducting qPCR reactions. This work was supported by grants from the Canadian Institutes for Health Research to F.S. (MOP-36399) and from the Canadian Cancer Society to M.T. (Award 018046). T.R. is a Research Fellow of The Terry Fox Foundation (Award 019684). F.S. is a recipient of a National Cancer Institute of Canada Scientist award, and M.T. is a Canada Research Chair (Tier II) in Intracellular Signaling.
Author Contributions T.R., M.S., F.S. and M.T. designed the study. T.R., M.S. and M.L. performed experiments. T.R. and M.S. analysed experiments. T.R., F.S. and M.T. wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
This file contains Supplementary Table 1, Supplementary Figures 1-9 with Legends, Supplementary Methods and Supplementary References. (PDF 3541 kb)
Rights and permissions
About this article
Cite this article
Rajakulendran, T., Sahmi, M., Lefrançois, M. et al. A dimerization-dependent mechanism drives RAF catalytic activation. Nature 461, 542–545 (2009). https://doi.org/10.1038/nature08314
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08314
This article is cited by
-
Live-cell target engagement of allosteric MEKi on MEK–RAF/KSR–14-3-3 complexes
Nature Chemical Biology (2024)
-
Targeting CRAF kinase in anti-cancer therapy: progress and opportunities
Molecular Cancer (2023)
-
Analysis of RAS and drug induced homo- and heterodimerization of RAF and KSR1 proteins in living cells using split Nanoluc luciferase
Cell Communication and Signaling (2023)
-
Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies
Signal Transduction and Targeted Therapy (2023)
-
Interactome dynamics of RAF1-BRAF kinase monomers and dimers
Scientific Data (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.