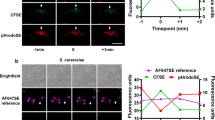
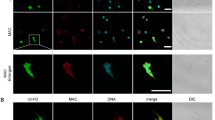
Abstract
Macrophages are aptly positioned to function as the primary line of defence against invading pathogens in many organs, including the lung and peritoneum. Their ability to phagocytose and clear microorganisms has been well documented1,2. Macrophages possess several substances with which they can kill bacteria, including reactive oxygen species, nitric oxide, and antimicrobial proteins3,4,5,6,7,8,9. We proposed that macrophage-derived proteinases may contribute to the antimicrobial properties of macrophages. Macrophage elastase (also known as matrix metalloproteinase 12 or MMP12) is an enzyme predominantly expressed in mature tissue macrophages10 and is implicated in several disease processes, including emphysema11. Physiological functions for MMP12 have not been described. Here we show that Mmp12-/- mice exhibit impaired bacterial clearance and increased mortality when challenged with both Gram-negative and Gram-positive bacteria at macrophage-rich portals of entry, such as the peritoneum and lung. Intracellular stores of MMP12 are mobilized to macrophage phagolysosomes after the ingestion of bacterial pathogens. Once inside phagolysosomes, MMP12 adheres to bacterial cell walls where it disrupts cellular membranes resulting in bacterial death. The antimicrobial properties of MMP12 do not reside within its catalytic domain, but rather within the carboxy-terminal domain. This domain contains a unique four amino acid sequence on an exposed β loop of the protein that is required for the observed antimicrobial activity. The present study represents, to our knowledge, the first report of direct antimicrobial activity by a matrix metallopeptidase, and describes a new antimicrobial peptide that is sequentially and structurally unique in nature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Green, G. M. & Kass, E. H. The role of the alveolar macrophage in the clearance of bacteria from the lung. J. Exp. Med. 119, 167–176 (1964)
Gordon, S. The macrophage: Past, present and future. Eur. J. Immunol. 37, S9–S17 (2007)
Shiloh, M. U. et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10, 29–38 (1999)
Ganz, T. Oxygen-independent microbicidal mechanisms of phagocytes. Proc. Am. Assoc. Physicians 111, 390–395 (1999)
Selsted, M. E. & Ouellette, A. J. Defensins in granules of phagocytic and non-phagocytic cells. Trends Cell Biol. 5, 114–119 (1995)
Biggar, W. D. & Sturgess, J. M. Role of lysozyme in the microbicidal activity of rat alveolar macrophages. Infect. Immun. 16, 974–982 (1977)
del Cerro-Vadillo, E. et al. Cutting edge: a novel nonoxidative phagosomal mechanism exerted by cathepsin-D controls Listeria monocytogenes intracellular growth. J. Immunol. 176, 1321–1325 (2006)
Hiemstra, P. S., van den Barselaar, M. T., Roest, M., Nibbering, P. H. & van Furth, R. Ubiquicidin, a novel murine microbicidal protein present in the cytosolic fraction of macrophages. J. Leukoc. Biol. 66, 423–428 (1999)
Hiemstra, P. S. et al. Antimicrobial proteins of murine macrophages. Infect. Immun. 61, 3038–3046 (1993)
Shipley, J. M., Wesselschmidt, R. L., Kobayashi, D. K., Ley, T. J. & Shapiro, S. D. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc. Natl Acad. Sci. USA 93, 3942–3946 (1996)
Hautamaki, R. D., Kobayashi, D. K., Senior, R. M. & Shapiro, S. D. Macrophage elastase is required for cigarette smoke-induced emphysema in mice. Science 277, 2002–2004 (1997)
Brinckerhoff, C. E. & Matrisian, L. M. Matrix metalloproteinases: a tail of a frog that became a prince. Nature Rev. Mol. Cell Biol. 3, 207–214 (2002)
Raza, S. L., Nehring, L. C., Shapiro, S. D. & Cornelius, L. A. Proteinase-activated receptor-1 regulation of macrophage elastase (MMP-12) secretion by serine proteinases. J. Biol. Chem. 275, 41243–41250 (2000)
Lijnen, H. R. et al. Temporal and topographic matrix metalloproteinase expression after vascular injury in mice. Thromb. Haemost. 81, 799–807 (1999)
Knauper, V. et al. The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J. Biol. Chem. 272, 7608–7616 (1997)
Tam, E. M., Moore, T. R., Butler, G. S. & Overall, C. M. Characterization of the distinct collagen binding, helicase and cleavage mechanisms of matrix metalloproteinase 2 and 14 (gelatinase A and MT1-MMP): the differential roles of the MMP hemopexin c domains and the MMP-2 fibronectin type II modules in collagen triple helicase activities. J. Biol. Chem. 279, 43336–43344 (2004)
McQuibban, G. A. et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J. Biol. Chem. 276, 43503–43508 (2001)
McQuibban, G. A. et al. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 289, 1202–1206 (2000)
Gronski, T. J. et al. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J. Biol. Chem. 272, 12189–12194 (1997)
Belaaouaj, A. et al. Mice lacking neutrophil elastase reveal impaired host defense against gram-negative bacterial sepsis. Nature Med. 4, 615–618 (1998)
Belaaouaj, A., Kim, K. S. & Shapiro, S. D. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science 289, 1185–1188 (2000)
Weinrauch, Y., Drujan, D., Shapiro, S., Weiss, J. & Zychlinsky, A. Neutrophil elastase targets virulence factors of enterobacteria. Nature 417, 91–94 (2002)
Lehrer, R. I. & Ganz, T. Antimicrobial polypeptides of human neutrophils. Blood 76, 2169–2181 (1990)
Wilson, C. L. et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286, 113–117 (1999)
Vu, T. H. et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93, 411–422 (1998)
Li, Q., Park, P., Wilson, C. & Parks, W. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111, 635–646 (2002)
Curci, J. A., Liao, S., Huffman, M. D., Shapiro, S. D. & Thompson, R. W. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J. Clin. Invest. 102, 1900–1910 (1998)
Heymans, S. et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture by impairs therapeutic angiogenesis and causes cardiac failure. Nature Med. 5, 1135–1142 (1999)
Golubkov, V. S. et al. Membrane type-1 matrix metalloproteinase (MT1-MMP- exhibits an important intracellular cleavage function and causes chromosome instability. J. Biol. Chem. 280, 25079–25086 (2005)
Limb, G. A. et al. Matrix metalloprteinase-1 associates with intracellular organelles and confers resistance to laminin a/c degradation during apoptosis. Am. J. Pathol. 166, 1555–1563 (2005)
Acknowledgements
This work was supported by grants from the National Institutes of Health (NIH) National Heart, Lung and Blood Institute (NHLBI) (S.D.S.) and Spanish and European public agencies (F.X.G.-R.).
Author Contributions A.M.H. performed in vivo and in vitro studies, contributed to data interpretation and mechanistic advance, prepared the manuscript and figures, and performed all revisions. W.O.H. performed in vivo and in vitro studies and contributed to study design, data analysis, and mechanistic advance. C.S.R. performed CTD processing studies. F.X.G.-R. constructed the three-dimensional homology model of MMP12 CTD. S.D.S. generated Mmp12-/- mice and all recombinant proteins, was responsible for study design, data interpretation, and mechanistic advance, and assisted with manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Houghton, A., Hartzell, W., Robbins, C. et al. Macrophage elastase kills bacteria within murine macrophages. Nature 460, 637–641 (2009). https://doi.org/10.1038/nature08181
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08181
This article is cited by
-
The aberrant cross-talk of epithelium–macrophages via METTL3-regulated extracellular vesicle miR-93 in smoking-induced emphysema
Cell Biology and Toxicology (2022)
-
ADAM15 expression is increased in lung CD8+ T cells, macrophages, and bronchial epithelial cells in patients with COPD and is inversely related to airflow obstruction
Respiratory Research (2020)
-
MMP12 Inhibits Corneal Neovascularization and Inflammation through Regulation of CCL2
Scientific Reports (2019)
-
C-terminal truncation of IFN-γ inhibits proinflammatory macrophage responses and is deficient in autoimmune disease
Nature Communications (2018)
-
Both Drosophila matrix metalloproteinases have released and membrane-tethered forms but have different substrates
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.