Abstract
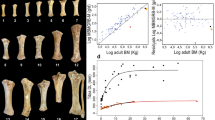
Body size reduction in mammals is usually associated with only moderate brain size reduction, because the brain and sensory organs complete their growth before the rest of the body during ontogeny1,2. On this basis, ‘phyletic dwarfs’ are predicted to have a greater relative brain size than ‘phyletic giants’1,3. However, this trend has been questioned in the special case of dwarfism of mammals on islands4. Here we show that the endocranial capacities of extinct dwarf species of hippopotamus from Madagascar are up to 30% smaller than those of a mainland African ancestor scaled to equivalent body mass. These results show that brain size reduction is much greater than predicted from an intraspecific ‘late ontogenetic’ model of dwarfism in which brain size scales to body size with an exponent of 0.35. The nature of the proportional change or grade shift2,5 observed here indicates that selective pressures on brain size are potentially independent of those on body size. This study demonstrates empirically that it is mechanistically possible for dwarf mammals on islands to evolve significantly smaller brains than would be predicted from a model of dwarfing based on the intraspecific scaling of the mainland ancestor. Our findings challenge current understanding of brain–body allometric relationships in mammals and suggest that the process of dwarfism could in principle explain small brain size, a factor relevant to the interpretation of the small-brained hominin found on the Island of Flores, Indonesia6.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Shea, B. T. Phyletic size change and brian/body allometry: a consideration based on the African pongids and other primates. Int. J. Primatol. 4, 33–62 (1983)
Martin, R. D. Human Brain Evolution in an Ecological Context: 52nd James Arthur Lecture on the Evolution of the Human Brain (American Museum of Natural History, New York, 1983)
Gould, S. J. Allometry in primates with emphasis on scaling and the evolution of the brain. Contrib. Primatol. 5, 244–292 (1975)
Köhler, M. & Moyà-Solà, S. Reduction of brain and sense organs in the fossil insular bovid Myotragus . Brain Behav. Evol. 63, 125–140 (2004)
Martin, R. D. & Harvey, P. H. in Size and Scaling in Primate Biology (ed. Jungers, W. L.) 147–173 (Plenum, 1985)
Brown, P. et al. A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature 431, 1055–1061 (2004)
Niven, J. E. Brains, islands and evolution: breaking all the rules. Trends Ecol. Evol. 22, 57–59 (2006)
Richards, G. D. Genetic, physiologic and ecogeographic factors contributing to variation in Homo sapiens: Homo floresiensis reconsidered. J. Evol. Biol. 19, 1744–1767 (2006)
Lalueza-Fox, C., Shapiro, B., Bover, P., Alcover, J. A. & Bertranpetit, J. Molecular phylogeny and evolution of the extinct bovid Myotragus balearicus . Mol. Phylogenet. Evol. 25, 501–510 (2002)
Martin, R. D. et al. Comment on ‘The brain of LB1, Homo floresiensis’. Science 312, 999b (2006)
Martin, R. D., Maclarnon, A. M., Phillips, J. L. & Dobyns, W. B. Flores hominid: new species or microcephalic dwarf? Anat. Rec. 288A, 1123–1145 (2006)
Köhler, M., Moyà-Solà, S. & Wrangham, R. W. Island rules cannot be broken. Trends Ecol. Evol. 23, 6–7 (2008)
Argue, D., Donlon, D., Groves, C. & Wright, R. Homo floresiensis: microcephalic, pygmoid, Australopithecus, or Homo? J. Hum. Evol. 51, 360–374 (2006)
Niven, J. E. Response to Köhler et al. Impossible arguments about possible species? Trends Ecol. Evol. 23, 8–9 (2008)
Lande, R. Quantitative genetic analysis of multivariate evolution, applied to brain:body size allometry. Evolution Int. J. Org. Evolution 33, 402–416 (1979)
Kruska, D. C. T. On the evolutionary significance of encephalization in some eutherian mammals: effects of adaptive radiation, domestication and feralization. Brain Behav. Evol. 65, 73–108 (2005)
Stuenes, S. Taxonomy, habits, and relationships of the subfossil Madagascan hippopoptami Hippopotamus lemerlei and H. madagascariensis . J. Vertebr. Paleontol. 9, 241–268 (1989)
Boisserie, J.-R. The phylogeny and taxonomy of Hippopotamidae (Mammalia: Artiodactyla): a review based on morphology and cladistic analysis. Zool. J. Linn. Soc. 143, 1–26 (2005)
Eltringham, S. K. The Hippos: Natural History and Conservation (Academic, 1999)
Burney, D. A. et al. A chronology for late prehistoric Madagascar. J. Hum. Evol. 47, 25–63 (2004)
Weston, E. M. Evolution of ontogeny in the hippopotamus skull: using allometry to dissect developmental change. Biol. J. Linn. Soc. 80, 625–638 (2003)
Accordi, F. S. & Palombo, M. R. Morfologia endocranica degli elefanti nani pleistocenici di Spinagallo (Siracusa) e comparazione con l’ endocranio di Elephas antiquus . Atti Accad. Naz. Lincei Rc. 51, 111–124 (1971)
Roth, V. L. Inferences from allometry and fossils: dwarfing of elephants on islands. Oxf. Surv. Evol. Biol. 8, 259–288 (1992)
Shoshani, J., Kupsky, W. J. & Marchant, G. H. Elephant brain. Part I: Gross morphology, functions, comparative anatomy, and evolution. Brain Res. Bull. 70, 124–157 (2006)
Christiansen, P. Body size in proboscideans, with notes on elephant metabolism. Zool. J. Linn. Soc. 140, 524–549 (2004)
Kappelman, J. The evolution of body mass and relative brain size in fossil hominids. J. Hum. Evol. 30, 243–276 (1996)
Stanyon, R., Consigliere, S. & Morescalchi, M. A. Cranial capacity in hominid evolution. Hum. Evol. 8, 205–216 (1993)
Jacob, T. et al. Pygmoid Australomelanesian Homo sapiens skeletal remains from Liang Bua, Flores: population affinities and pathological abnormalities. Proc. Natl Acad. Sci. USA 103, 13421–13426 (2006)
Lordkipanidze, D. et al. A fourth hominin skull from Dmanisi, Georgia. Anat. Rec. 288A, 1146–1157 (2006)
Lordkipanidze, D. et al. Postcranial evidence from early Homo from Dmanisi, Georgia. Nature 449, 305–310 (2007)
Acknowledgements
We thank A. Currant, C. Lefèvre, C. Sagne, E. Gilissen, F. Renoult, H. Chatterjee, J. Ashby, M. Nowak-Kemp, M. Harman, P. Jenkins, P. Tassy, R. Sabin, R. Symonds and S. Stuenes for facilitating access to museum collections; A. Rasoamiaramanana, G. Ravololonarivo, H. Andriamialison, T. Rakotondrazafy, M. Ramarolahy and S. Bourlat for permission and/or assistance with study of the subfossil material held in the University of Antananarivo and the Académie Malagache; B. Ramanivosoa, D. Gommery, C. Guérin and M. Faure for allowing the study of material at the Akiba Museum, Mahajanga, Madagascar; R. Portela Miguez for assistance with recording endocranial capacity measures from H. amphibius specimens in the Natural History Museum, London; A. Friday for assistance with data collection in the University Museum of Zoology, Cambridge; C. Anderung, J.-R. Boisserie, S. Walsh and V. Herridge for discussion and helpful comments; and J. Kappelman, J. Niven, D. Lieberman and A. Gordon for comments on earlier versions of this manuscript. This research was supported by the Biotechnology and Biological Sciences Research Council.
Author Contributions E.W. and A.L. designed the study. E.W. collected and analysed the data and drafted the paper. Both authors discussed the results and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Data, Supplementary Tables 1-5, Supplementary Figures 1-8 with Legends a Supplementary Discussion and Supplementary References. (PDF 1534 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Weston, E., Lister, A. Insular dwarfism in hippos and a model for brain size reduction in Homo floresiensis. Nature 459, 85–88 (2009). https://doi.org/10.1038/nature07922
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07922
This article is cited by
-
Allometric brain reduction in an insular, dwarfed population of black-tailed deer
Journal of Mammalian Evolution (2023)
-
Skeleton of a Cretaceous mammal from Madagascar reflects long-term insularity
Nature (2020)
-
From Jumbo to Dumbo: Cranial Shape Changes in Elephants and Hippos During Phyletic Dwarfing
Evolutionary Biology (2018)
-
The effect of body size evolution and ecology on encephalization in cave bears and extant relatives
BMC Evolutionary Biology (2017)
-
Seasonality and brain size are negatively associated in frogs: evidence for the expensive brain framework
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.