Abstract
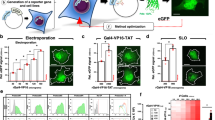
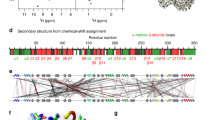
Investigating proteins ‘at work’ in a living environment at atomic resolution is a major goal of molecular biology, which has not been achieved even though methods for the three-dimensional (3D) structure determination of purified proteins in single crystals or in solution are widely used. Recent developments in NMR hardware and methodology have enabled the measurement of high-resolution heteronuclear multi-dimensional NMR spectra of macromolecules in living cells (in-cell NMR)1,2,3,4,5. Various intracellular events such as conformational changes, dynamics and binding events have been investigated by this method. However, the low sensitivity and the short lifetime of the samples have so far prevented the acquisition of sufficient structural information to determine protein structures by in-cell NMR. Here we show the first, to our knowledge, 3D protein structure calculated exclusively on the basis of information obtained in living cells. The structure of the putative heavy-metal binding protein TTHA1718 from Thermus thermophilus HB8 overexpressed in Escherichia coli cells was solved by in-cell NMR. Rapid measurement of the 3D NMR spectra by nonlinear sampling of the indirectly acquired dimensions was used to overcome problems caused by the instability and low sensitivity of living E. coli samples. Almost all of the expected backbone NMR resonances and most of the side-chain NMR resonances were observed and assigned, enabling high quality (0.96 ångström backbone root mean squared deviation) structures to be calculated that are very similar to the in vitro structure of TTHA1718 determined independently. The in-cell NMR approach can thus provide accurate high-resolution structures of proteins in living environments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Protein Data Bank
Data deposits
Atomic coordinates of the structures of TTHA1718 in E. coli cells and in vitro have been deposited in the Protein Data Bank under accession codes 2ROG and 2ROE, respectively. Chemical shifts have been deposited in the BioMagResBank under accession numbers 11037 and 11035.
References
Serber, Z. et al. High-resolution macromolecular NMR spectroscopy inside living cells. J. Am. Chem. Soc. 123, 2446–2447 (2001)
Serber, Z., Corsini, L., Durst, F. & Dötsch, V. In-cell NMR spectroscopy. Methods Enzymol. 394, 17–41 (2005)
Serber, Z. et al. Investigating macromolecules inside cultured and injected cells by in-cell NMR spectroscopy. Nature Protocols 1, 2701–2709 (2006)
Reckel, S., Hänsel, R., Löhr, F. & Dötsch, V. In-cell NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 51, 91–101 (2007)
Selenko, P. & Wagner, G. Looking into live cells with in-cell NMR spectroscopy. J. Struct. Biol. 158, 244–253 (2007)
Ellis, R. J. Macromolecular crowding: obvious but underappreciated. Trends Biochem. Sci. 26, 597–604 (2001)
Burz, D. S., Dutta, K., Cowburn, D. & Shekhtman, A. Mapping structural interactions using in-cell NMR spectroscopy (STINT-NMR). Nature Methods 3, 91–93 (2006)
McNulty, B. C., Young, G. B. & Pielak, G. J. Macromolecular crowding in the Escherichia coli periplasm maintains α-synuclein disorder. J. Mol. Biol. 355, 893–897 (2006)
Dedmon, M. M., Patel, C. N., Young, G. B. & Pielak, G. J. FlgM gains structure in living cells. Proc. Natl Acad. Sci. USA 99, 12681–12684 (2002)
Selenko, P., Serber, Z., Gade, B., Ruderman, J. & Wagner, G. Quantitative NMR analysis of the protein G B1 domain in Xenopus laevis egg extracts and intact oocytes. Proc. Natl Acad. Sci. USA 103, 11904–11909 (2006)
Sakai, T. et al. In-cell NMR spectroscopy of proteins inside Xenopus laevis oocytes. J. Biomol. NMR 36, 179–188 (2006)
Inomata, K. et al. High-resolution multi-dimensional NMR spectroscopy of proteins in living human cells. Nature 10.1038/nature07839 (this issue)
Wüthrich, K. NMR of Proteins and Nucleic Acids (Wiley, 1986)
Reardon, P. N. & Spicer, L. D. Multidimensional NMR spectroscopy for protein characterization and assignment inside cells. J. Am. Chem. Soc. 127, 10848–10849 (2005)
Serber, Z., Ledwidge, R., Miller, S. M. & Dötsch, V. Evaluation of parameters critical to observing proteins inside living Escherichia coli by in-cell NMR spectroscopy. J. Am. Chem. Soc. 123, 8895–8901 (2001)
Barna, J. C. J., Laue, E. D., Mayger, M. R., Skilling, J. & Worrall, S. J. P. Exponential sampling, an alternative method for sampling in two-dimensional NMR experiments. J. Magn. Reson. 73, 69–77 (1987)
Schmieder, P., Stern, A. S., Wagner, G. & Hoch, J. C. Improved resolution in triple-resonance spectra by nonlinear sampling in the constant-time domain. J. Biomol. NMR 4, 483–490 (1994)
Rovnyak, D. et al. Accelerated acquisition of high resolution triple-resonance spectra using non-uniform sampling and maximum entropy reconstruction. J. Magn. Reson. 170, 15–21 (2004)
Mueller, G. A. et al. Global folds of proteins with low densities of NOEs using residual dipolar couplings: application to the 370-residue maltodextrin-binding protein. J. Mol. Biol. 300, 197–212 (2000)
Serber, Z. et al. Methyl groups as probes for proteins and complexes in in-cell NMR experiments. J. Am. Chem. Soc. 126, 7119–7125 (2004)
Rosen, M. K. et al. Selective methyl group protonation of perdeuterated proteins. J. Mol. Biol. 263, 627–636 (1996)
Güntert, P., Mumenthaler, C. & Wüthrich, K. Torsion angle dynamics for NMR structure calculation with the new program DYANA. J. Mol. Biol. 273, 283–298 (1997)
Kainosho, M. et al. Optimal isotope labelling for NMR protein structure determinations. Nature 440, 52–57 (2006)
Li, C. et al. Differential dynamical effects of macromolecular crowding on an intrinsically disordered protein and a globular protein: implications for in-cell NMR spectroscopy. J. Am. Chem. Soc. 130, 6310–6311 (2008)
Kraulis, P. J., Domaille, P. J., Campbell-Burk, S. L., Van Aken, T. & Laue, E. D. Solution structure and dynamics of Ras p21-GDP determined by heteronuclear three- and four-dimensional NMR spectroscopy. Biochemistry 33, 3515–3531 (1994)
Laue, E. D., Mayger, M. R., Skilling, J. & Staunton, J. Reconstruction of phase sensitive 2D NMR spectra by maximum entropy. J. Magn. Reson. 68, 14–29 (1986)
Herrmann, T., Güntert, P. & Wüthrich, K. Protein NMR structure determination with automated NOE assignment using the new software CANDID and the torsion angle dynamics algorithm DYANA. J. Mol. Biol. 319, 209–227 (2002)
Cornilescu, G., Delaglio, F. & Bax, A. Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J. Biomol. NMR 13, 289–302 (1999)
Cornell, W. D. et al. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J. Am. Chem. Soc. 117, 5179–5197 (1995)
Koradi, R., Billeter, M. & Güntert, P. Point-centered domain decomposition for parallel molecular dynamics simulation. Comput. Phys. Commun. 124, 139–147 (2000)
Gardy, J. L. et al. PSORT-B: Improving protein subcellular localization prediction for gram-negative bacteria. Nucleic Acids Res. 31, 3613–3617 (2003)
Gardy, J. L. et al. PSORTb v.2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics 21, 617–623 (2005)
Nielsen, H., Engelbrecht, J., Brunak, S. & von Heijne, G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10, 1–6 (1997)
Bendtsen, J. D., Nielsen, H., von Heijne, G. & Brunak, S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340, 783–795 (2004)
Thorstenson, Y. R., Zhang, Y., Olson, P. S. & Mascarenhas, D. Leaderless polypeptides efficiently extracted from whole cells by osmotic shock. J. Bacteriol. 179, 5333–5339 (1997)
Hayashi, N., Matsubara, M., Takasaki, A., Titani, K. & Taniguchi, H. An expression system of rat calmodulin using T7 phage promoter in Escherichia coli. Protein Expr. Purif. 12, 25–28 (1998)
Kraulis, P. J. ANSIG: a program for the assignment of protein 1H 2D NMR spectra by interactive computer graphics. J. Magn. Reson. 84, 627–633 (1989)
Güntert, P. Automated NMR protein structure calculation. Prog. Nucl. Magn. Reson. Spectrosc. 43, 105–125 (2003)
Acknowledgements
The authors thank S. Kuramitsu for providing the plasmid encoding TTHA1718, and D. Nietlispach for setting up 3D NMR experiments with nonlinear sampling schemes and 15N relaxation experiments, T. Anzai for assistance with NMR measurements, and H. Koyama and A. Iwasaki for sample preparations. This work was supported in part by CREST, Japan Science and Technology Agency (JST), the Molecular Ensemble Program, RIKEN, Grants-in-Aid for Scientific Research of Priority Areas from the Japanese Ministry of Education, Sports, Culture, Science, and Technology on ‘Molecular Soft Interactions Regulating Membrane Interface of Biological Systems’ and ‘Molecular Science for Supra Functional Systems – Development of Advanced Methods for Exploring Elementary Process’, and by the Volkswagen Foundation.
Author Contributions B.O.S., M.S., P.G. and Y.I. designed the research and wrote the manuscript. D.S., A.S. and T.I. conducted the research including sample preparation, data acquisition, resonance assignment and structure calculation. M.M. and M.W. helped with NMR measurements. M.M. prepared TTHA1718 mutants. J.H. and T.H. measured NMR data on TTHA1718 mutants and 15N-relaxation experiments. N.H. provided the expression vector for calmodulin. M.Y. measured NMR data on calmodulin in living E. coli cells. T.M. helped during the preparation and characterisation of TTHA1718.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-11 with Legends and Supplementary Tables 1-2 (PDF 5215 kb)
Rights and permissions
About this article
Cite this article
Sakakibara, D., Sasaki, A., Ikeya, T. et al. Protein structure determination in living cells by in-cell NMR spectroscopy. Nature 458, 102–105 (2009). https://doi.org/10.1038/nature07814
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07814
This article is cited by
-
AI-designed NMR spectroscopy RF pulses for fast acquisition at high and ultra-high magnetic fields
Nature Communications (2023)
-
Protein structure determination in human cells by in-cell NMR and a reporter system to optimize protein delivery or transexpression
Communications Biology (2022)
-
Microfluidics delivery of DARPP-32 into HeLa cells maintains viability for in-cell NMR spectroscopy
Communications Biology (2022)
-
NMR refinement and peptide folding using the GROMACS software
Journal of Biomolecular NMR (2021)
-
Protein in-cell NMR spectroscopy at 1.2 GHz
Journal of Biomolecular NMR (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.