Abstract
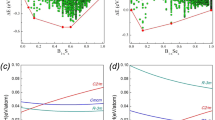
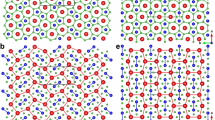
Boron is an element of fascinating chemical complexity. Controversies have shrouded this element since its discovery was announced in 1808: the new ‘element’ turned out to be a compound containing less than 60–70% of boron, and it was not until 1909 that 99% pure boron was obtained1. And although we now know of at least 16 polymorphs2, the stable phase of boron is not yet experimentally established even at ambient conditions3. Boron’s complexities arise from frustration: situated between metals and insulators in the periodic table, boron has only three valence electrons, which would favour metallicity, but they are sufficiently localized that insulating states emerge. However, this subtle balance between metallic and insulating states is easily shifted by pressure, temperature and impurities. Here we report the results of high-pressure experiments and ab initio evolutionary crystal structure predictions4,5 that explore the structural stability of boron under pressure and, strikingly, reveal a partially ionic high-pressure boron phase. This new phase is stable between 19 and 89 GPa, can be quenched to ambient conditions, and has a hitherto unknown structure (space group Pnnm, 28 atoms in the unit cell) consisting of icosahedral B12 clusters and B2 pairs in a NaCl-type arrangement. We find that the ionicity of the phase affects its electronic bandgap, infrared adsorption and dielectric constants, and that it arises from the different electronic properties of the B2 pairs and B12 clusters and the resultant charge transfer between them.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Petryanov-Sokolov, I. V. (ed.) Popular Library of the Elements Ch. 5 (Nauka, 1983)
Douglas, B. E. & Ho, S.-M. Structure and Chemistry of Crystalline Solids (Springer, 2006)
Chase, M. W. J. Phys. Chem. Ref. Data 9, (1998)
Oganov, A. R. & Glass, C. W. Crystal structure prediction using ab initio evolutionary algorithms: Principles and applications. J. Chem. Phys. 124, 244704 (2006)
Glass, C. W., Oganov, A. R. & Hansen, N. USPEX — evolutionary crystal structure prediction. Comput. Phys. Commun. 175, 713–720 (2006)
Wells, A. F. Structural Inorganic Chemistry (Clarendon, 1986)
Amberger, E. & Ploog, K. Bildung der Gitter des Reinen Bors. J. Less Common Met. 23, 21–31 (1971)
Vlasse, M., Naslain, R., Kasper, J. S. & Ploog, K. Crystal structure of tetragonal boron related to α-AlB12 . J. Solid State Chem. 28, 289–301 (1979)
Masago, A., Shirai, K. & Katayama-Yoshida, H. Crystal stability of α- and β-boron. Phys. Rev. B 73, 104102 (2006)
van Setten, M. J., Uijttewaal, M. A., de Wijs, G. A. & de Groot, R. A. Thermodynamic stability of boron: The role of defects and zero point motion. J. Am. Chem. Soc. 129, 2458–2465 (2007)
Sanz, D. N., Loubeyre, P. & Mezouar, M. Equation of state and pressure induced amorphization of β-boron from X-ray measurements up to 100 GPa. Phys. Rev. Lett. 89, 245501 (2002)
Häussermann, U., Simak, S. I., Ahuja, R. & Johansson, B. Metal-nonmetal transition in the boron group elements. Phys. Rev. Lett. 90, 065701 (2003)
Eremets, M. I., Struzhkin, V. W., Mao, H. K. & Hemley, R. J. Superconductivity in boron. Science 293, 272–274 (2001)
Ma, Y. Z., Prewitt, C. T., Zou, G. T., Mao, H. K. & Hemley, R. J. High-pressure high-temperature x-ray diffraction of β-boron to 30 GPa. Phys. Rev. B 67, 174116 (2003)
McMahon, M. I. & Nelmes, R. J. High-pressure structures and phase transformations in elemental metals. Chem. Soc. Rev. 35, 943–963 (2006)
Bader, R. F. W. Atoms in Molecules. A Quantum Theory (Oxford Univ. Press, 1990)
Edwards, B. & Ashcroft, N. W. Spontaneous polarization in dense hydrogen. Nature 388, 652–655 (1997)
Hayami, W. Theoretical study of the stability of AB12 (A = H-Ne) icosahedral clusters. Phys. Rev. B 60, 1523–1526 (1999)
Werheit, H., Luax, M. & Kuhlmann, U. Interband and gap state related transitions in β-rhombohedral boron. Phys. Status Solidi B 176, 415–432 (1993)
Ma, Y. M., Tse, J. S., Klug, D. D. & Ahuja, R. Electron-phonon coupling of α-Ga boron. Phys. Rev. B 70, 214107 (2004)
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996)
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994)
Kresse, G. & Furthmüller, J. Efficiency of ab initio total-energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996)
Baroni, S., de Gironcoli, S., Dal Corso, A. & Gianozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 73, 515–562 (2001)
Gonze, X. et al. First-principles computation of materials properties: The ABINIT software project. Comput. Mater. Sci. 25, 478–492 (2002)
Dovesi, R. et al. CRYSTAL06 User's Manual (University of Torino, 2006)
Solozhenko, V. L., Le Godec, Y. & Kurakevych, O. O. Solid-state synthesis of boron subnitride, B6N: myth or reality? C.R. Chimie 9, 1472–1475 (2006)
Will, G. & Kiefer, B. Electron deformation density in α-boron. Z. Anorg. Allg. Chem. 627, 2100–2104 (2001)
Brazhkin, V. V., Taniguchi, T., Akaishi, M. & Popova, S. V. Fabrication of β-boron by chemical-reaction and melt-quenching methods at high pressures. J. Mater. Res. 19, 1643–1648 (2004)
Acknowledgements
A.R.O. acknowledges the Swiss National Science Foundation (grant 200021-111847/1) and the ETH Research Equipment Programme for support of this work. J.C. and T.Y. were supported by the NSF (grant EAR0711321) and the DOE (contract DE-FG02-07ER46461), Yanzhang Ma was funded by the DOE (agreement DE-FC03-03NA00144) and the NSF (grant DMR-0619215), and O.O.K. and V.L.S. were supported by the Agence Nationale de la Recherche (grant ANR-05-BLAN-0141). The use of the NSLS at Brookhaven National Laboratory was supported by the US Department of Energy under contract DE-AC02-98CH10886, and high pressure beamlines at the NSLS were supported by COMPRES under NSF cooperative agreement EAR 06-49658. Calculations were performed at CSCS (Manno), ETH Zurich, and the Joint Supercomputer Centre of the Russian Academy of Sciences.
Author Contributions A.R.O. did most of the calculations and wrote most of the paper, C.G. did most of the analysis of chemical bonding and wrote a significant part of the discussion, Yanming Ma contributed to calculations, C.W.G. wrote the first version of the USPEX code, J.C., V.L.S. and Yanzhang Ma synthesized the new phase, J.C. performed infrared absorption measurements with Z.L. and T.Y., V.L.S. did the Raman measurements and elemental analysis, V.L.S. and Yanzhang Ma did synchrotron X-ray diffraction measurements, and J.C. and O.O.K. performed the Le Bail refinement of the X-ray diffraction patterns.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Data and Methods, Supplementary Figures S1-S5 with Legends, Supplementary Tables S1- S6 and Supplementary References (PDF 1491 kb)
Rights and permissions
About this article
Cite this article
Oganov, A., Chen, J., Gatti, C. et al. Ionic high-pressure form of elemental boron. Nature 457, 863–867 (2009). https://doi.org/10.1038/nature07736
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07736
This article is cited by
-
Prediction of properties of boron \(\alpha\)-icosahedral nanosheet by bond-addictive \({\mathbb {M}}\)-polynomial
Scientific Reports (2024)
-
Two-dimensional ferroelectricity in a single-element bismuth monolayer
Nature (2023)
-
Emerging monoelemental 2D materials (Xenes) for biosensor applications
Nano Research (2023)
-
Possible boron-rich amorphous silicon borides from ab initio simulations
Journal of Molecular Modeling (2023)
-
Superatomic icosahedral-CnB12-n (n = 0, 1, 2) Stuffed mononuclear and binuclear borafullerene and borospherene nanoclusters with spherical aromaticity
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.