Abstract
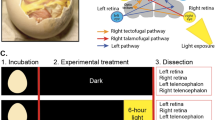
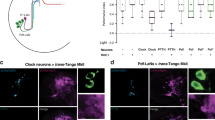
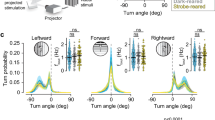
Specification of the appropriate neurotransmitter is a crucial step in neuronal differentiation because it enables signalling among populations of neurons. Experimental manipulations demonstrate that both autonomous and activity-dependent genetic programs contribute to this process during development, but whether natural environmental stimuli specify transmitter expression in a neuronal population is unknown. We investigated neurons of the ventral suprachiasmatic nucleus that regulate neuroendocrine pituitary function in response to light in teleosts, amphibia and primates. Here we show that altering light exposure, which changes the sensory input to the circuit controlling adaptation of skin pigmentation to background, changes the number of neurons expressing dopamine in larvae of the amphibian Xenopus laevis in a circuit-specific and activity-dependent manner. Neurons newly expressing dopamine then regulate changes in camouflage colouration in response to illumination. Thus, physiological activity alters the numbers of behaviourally relevant amine-transmitter-expressing neurons in the brain at postembryonic stages of development. The results may be pertinent to changes in cognitive states that are regulated by biogenic amines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Walicke, P. A. & Patterson, P. H. On the role of Ca2+ in the transmitter choice made by cultured sympathetic neurons. J. Neurosci. 1, 343–350 (1981)
Brosenitsch, T. A. & Katz, D. M. Expression of Phox2 transcription factors and induction of the dopaminergic phenotype in primary sensory neurons. Mol. Cell. Neurosci. 20, 447–457 (2002)
Borodinsky, L. N. et al. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature 429, 523–530 (2004)
Gomez-Lira, G., Lamas, M., Romo-Parra, H. & Gutierrez, R. Programmed and induced phenotype of the hippocampal granule cells. J. Neurosci. 25, 6939–6946 (2005)
Catalano, S. M., Chang, C. K. & Shatz, C. J. Activity-dependent regulation of NMDAR1 immunoreactivity in the developing visual cortex. J. Neurosci. 17, 8376–8390 (1997)
Kidd, F. L. & Isaac, J. T. R. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature 400, 569–573 (1999)
Shi, J., Townsend, M. & Constantine-Paton, M. Activity-dependent induction of tonic calcineurin activity mediates a rapid developmental downregulation of NMDA receptor currents. Neuron 28, 103–114 (2000)
Brunelli, G. et al. Glutamatergic reinnervation through peripheral nerve graft dictates assembly of glutamatergic synapses at rat skeletal muscle. Proc. Natl Acad. Sci. USA 102, 8752–8757 (2005)
Borodinsky, L. N. & Spitzer, N. C. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proc. Natl Acad. Sci. USA 104, 335–340 (2007)
Fletcher, C. F. et al. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell 87, 607–617 (1996)
Hess, E. J. & Wilson, M. C. Tottering and leaner mutations perturb transient developmental expression of tyrosine-hydroxylase in embryologically distinct Purkinje-cells. Neuron 6, 123–132 (1991)
Ubink, R., Tuinhof, R. & Roubos, E. W. Identification of suprachiasmatic melanotrope-inhibiting neurons in Xenopus laevis: A confocal laser-scanning microscopy study. J. Comp. Neurol. 397, 60–68 (1998)
Tuinhof, R. et al. Involvement of retinohypothalamic input, suprachiasmatic nucleus, magnocellular nucleus and locus-coeruleus in control of melanotrope cells of Xenopus-laevis — a retrograde and anterograde tracing study. Neuroscience 61, 411–420 (1994)
Kramer, B. M. R. et al. Dynamics and plasticity of peptidergic control centres in the retino–brain–pituitary system of Xenopus laevis . Microsc. Res. Tech. 54, 188–199 (2001)
Kolk, S. M., Berghs, C., Vaudry, H., Verhage, M. & Roubos, E. W. Physiological control of Xunc18 expression in neuroendocrine melanotrope cells of Xenopus laevis . Endocrinology 142, 1950–1957 (2001)
Abizaid, A., Horvath, B., Keefe, D. L., Leranth, C. & Horvath, T. L. Direct visual and circadian pathways target neuroendocrine cells in primates. Eur. J. Neurosci. 20, 2767–2776 (2004)
Logan, D. W., Burn, S. F. & Jackson, I. J. Regulation of pigmentation in zebrafish melanophores. Pigment Cell Res. 19, 206–213 (2006)
Roubos, E. W., Scheenen, W. & Jenks, B. G. in Trends in Comparative Endocrinology and Neurobiology 172–183. (2005)
Nordland, J. J. et al. The Pigmentary System: Physiology and Pathophysiology (Oxford Univ. Press, 2006)
Tonosaki, Y., Nishiyama, K., Honda, T., Ozaki, N. & Sugiura, Y. D-2-like dopamine-receptor mediates dopaminergic or gamma-aminobutyric acidergic inhibition of melanotropin-releasing hormone release from the pars intermedia in frogs (Rana-nigromaculata). Endocrinology 136, 5260–5265 (1995)
Akopian, A. & Witkovsky, P. D2 dopamine receptor-mediated inhibition of a hyperpolarization-activated current in rod photoreceptors. J. Neurophysiol. 76, 1828–1835 (1996)
Wang, Y., Harsanyi, K. & Mangel, S. C. Endogenous activation of dopamine D2 receptors regulates dopamine release in the fish retina. J. Neurophysiol. 78, 439–449 (1997)
Li, L. & Dowling, J. E. Effects of dopamine depletion on visual sensitivity of zebrafish. J. Neurosci. 20, 1893–1903 (2000)
Green, C. B., Liang, M. Y., Steenhard, B. M. & Besharse, J. C. Ontogeny of circadian and light regulation of melatonin release in Xenopus laevis embryos. Dev. Brain Res. 117, 109–116 (1999)
Mastick, G. S. & Andrews, G. L. Pax6 regulates the identity of embryonic diencephalic neurons. Mol. Cell. Neurosci. 17, 190–207 (2001)
Wullimann, M. F. & Rink, E. Detailed immunohistology of Pax6 protein and tyrosine hydroxylase in the early zebrafish brain suggests role of Pax6 gene in development of dopaminergic diencephalic neurons. Dev. Brain Res. 131, 173–191 (2001)
Vazquez-Martinez, R. et al. Melanotrope cell plasticity: a key mechanism for the physiological adaptation to background color changes. Endocrinology 142, 3060–3067 (2001)
Berghs, C., Tanaka, S., VanStrien, F. J. C., Kurabuchi, S. & Roubos, E. W. The secretory granule and pro-opiomelanocortin processing in Xenopus melanotrope cells during background adaptation. J. Histochem. Cytochem. 45, 1673–1682 (1997)
Zhang, H. et al. Calcium channel kinetics of melanotrope cells in Xenopus laevis depend on environmental stimulation. Gen. Comp. Endocrinol. 156, 104–112 (2008)
Jenks, B. G., Kidane, A. H., Scheenen, W. & Roubos, E. W. Plasticity in the melanotrope neuroendocrine interface of Xenopus laevis . Neuroendocrinology 85, 177–185 (2007)
Lam, C. S., Korzh, V. & Strahle, U. Zebrafish embryos are susceptible to the dopaminergic neurotoxin MPTP. Eur. J. Neurosci. 21, 1758–1762 (2005)
McKinley, E. T. et al. Neuroprotection of MPTP-induced toxicity in zebrafish dopaminergic neurons. Brain Res. Mol. Brain Res. 141, 128–137 (2005)
Olive, S., Rougon, G., Pierre, K. & Theodosis, D. T. Expression of a glycosyl phosphatidylinositol-anchored adhesion molecule, the glycoprotein F3, in the adult rat hypothalamoneurohypophyseal system. Brain Res. 689, 271–280 (1995)
El Majdoubi, M., Poulain, D. A. & Theodosis, D. T. Activity-dependent morphological synaptic plasticity in an adult neurosecretory system: magnocellular oxytocin neurons of the hypothalamus. Biochem. Cell Biol. 78, 317–327 (2000)
Mueller, N. K., Di, S., Paden, C. M. & Herman, J. P. Activity-dependent modulation of neurotransmitter innervation to vasopressin neurons of the supraoptic nucleus. Endocrinology 146, 348–354 (2005)
Froemke, R. C., Merzenich, M. M. & Schreiner, C. E. A synaptic memory trace for cortical receptive field plasticity. Nature 450, 425–429 (2007)
Lam, R. W. & Levitan, R. D. Pathophysiology of seasonal affective disorder: a review. J. Psychiatry Neurosci. 25, 469–480 (2000)
Lam, R. W. & Levitt, A. J. Canadian Consensus Guidelines for the Treatment of Seasonal Affective Disorder (Clinical and Academic Publishing, 1999)
Lam, R. W., Tam, E. M., Grewal, A. & Yatham, L. N. Effects of α-methyl-para-tyrosine-induced catecholamine depletion in patients with seasonal affective disorder in summer remission. Neuropsychopharmacology 25, S97–S101 (2001)
Michel, S., Itri, J. & Colwell, C. S. Excitatory mechanisms in the suprachiasmatic nucleus: the role of AMPA/KA glutamate receptors. J. Neurophysiol. 88, 817–828 (2002)
Baquet, Z. C., Bickford, P. C. & Jones, K. R. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compActa. J. Neurosci. 25, 6251–6259 (2005)
McFarlane, S., McNeill, L. & Holt, C. E. FGF signaling and target recognition in the developing Xenopus visual system. Neuron 15, 1017–1028 (1995)
Kim, J. et al. A microRNA feedback circuit in midbrain dopamine neurons. Science 317, 1220–1224 (2007)
Goridis, C. & Rohrer, H. Specification of catecholaminergic and serotonergic neurons. Nature Rev. Neurosci. 3, 531–541 (2002)
Obernosterer, G., Martinez, J. & Alenius, M. Locked nucleic acid-based in situ detection of microRNAs in mouse tissue sections. Nature Protocols 2, 1508–1514 (2007)
Gonzalez, A. & Smeets, W. Comparative analysis of dopamine and tyrosine hydroxylase immunoreactivities in the brain of 2 amphibians, the anuran Rana ridibunda and the urodele Pleurodeles waltlii . J. Comp. Neurol. 303, 457–477 (1991)
Acknowledgements
We thank D. Berg, L. Borodinsky and R. Levine for critical comments on the manuscript and I-T. Hsieh and D. Boassa for technical support. This work was supported by a grant to N.C.S. from the National Institutes of Health.
Author Contributions D.D. and N.C.S. planned the project, D.D. designed and carried out the experiments and performed data analysis, and D.D. and N.C.S. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information 1
This file contains Supplementary Figures 1-4, 6-12 and 14-17 with Legends. (PDF 10680 kb)
Supplementary Video 1
Supplementary Figure 5 is a video file illustrating calcium transients in the hypothalamus of the stage 35 control larva illustrated in Supplementary Figure 4. The video was generated by acquiring a Fluo-4 confocal image series (1 frame/5 sec) for 15 min and digitally resampling it to play 60 times faster. Calcium spikes occur with a15% incidence at this stage. (AVI 25855 kb)
Supplementary Video 2
Supplementary Figure 13 Following adaptation in the light for 2 hr, annular neurons, identified by NPY expression (red), co-express TH (green) in their somata and axonal projections (yellow) to the melanotrope cells. 3-D video of VSC reconstruction obtained by merging confocal stacks imaged through a brain wholemount from a white-adapted stage 42 larva. Scale bar: 50 µm. (AVI 35762 kb)
Rights and permissions
About this article
Cite this article
Dulcis, D., Spitzer, N. Illumination controls differentiation of dopamine neurons regulating behaviour. Nature 456, 195–201 (2008). https://doi.org/10.1038/nature07569
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07569
This article is cited by
-
N:ZnO/MoS2-heterostructured flexible synaptic devices enabling optoelectronic co-modulation for robust artificial visual systems
Nano Research (2024)
-
Neurotransmitter phenotype switching by spinal excitatory interneurons regulates locomotor recovery after spinal cord injury
Nature Neuroscience (2022)
-
High-frequency stimulation of the subthalamic nucleus induces a sustained inhibition of serotonergic system via loss of cell phenotype
Scientific Reports (2022)
-
Endoplasmic Reticulum in Metaplasticity: From Information Processing to Synaptic Proteostasis
Molecular Neurobiology (2022)
-
Developmental nicotine exposure affects larval brain size and the adult dopaminergic system of Drosophila melanogaster
BMC Developmental Biology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.