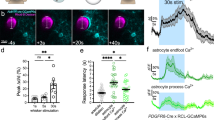
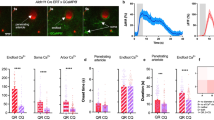
Abstract
Calcium signalling in astrocytes couples changes in neural activity to alterations in cerebral blood flow by eliciting vasoconstriction or vasodilation of arterioles. However, the mechanism for how these opposite astrocyte influences provide appropriate changes in vessel tone within an environment that has dynamic metabolic requirements remains unclear. Here we show that the ability of astrocytes to induce vasodilations over vasoconstrictions relies on the metabolic state of the rat brain tissue. When oxygen availability is lowered and astrocyte calcium concentration is elevated, astrocyte glycolysis and lactate release are maximized. External lactate attenuates transporter-mediated uptake from the extracellular space of prostaglandin E2, leading to accumulation and subsequent vasodilation. In conditions of low oxygen concentration extracellular adenosine also increases, which blocks astrocyte-mediated constriction, facilitating dilation. These data reveal the role of metabolic substrates in regulating brain blood flow and provide a mechanism for differential astrocyte control over cerebrovascular diameter during different states of brain activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mukamel, R. et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science 309, 951–954 (2005)
Zonta, M. et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature Neurosci. 6, 43–50 (2003)
Schummers, J., Yu, H. & Sur, M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320, 1638–1643 (2008)
Simard, M., Arcuino, G., Takano, T., Liu, Q. S. & Nedergaard, M. Signaling at the gliovascular interface. J. Neurosci. 23, 9254–9262 (2003)
Mulligan, S. J. & MacVicar, B. A. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431, 195–199 (2004)
Metea, M. R. & Newman, E. A. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J. Neurosci. 26, 2862–2870 (2006)
Chuquet, J., Hollender, L. & Nimchinsky, E. A. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J. Neurosci. 27, 4036–4044 (2007)
Filosa, J. A., Bonev, A. D. & Nelson, M. T. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ. Res. 95, e73–e81 (2004)
Takano, T. et al. Astrocyte-mediated control of cerebral blood flow. Nature Neurosci. 9, 260–267 (2006)
Filosa, J. A. et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nature Neurosci. 9, 1397–1403 (2006)
Mintun, M. A., Vlassenko, A. G., Rundle, M. M. & Raichle, M. E. Increased lactate/pyruvate ratio augments blood flow in physiologically activated human brain. Proc. Natl Acad. Sci. USA 101, 659–664 (2004)
Ido, Y., Chang, K. & Williamson, J. R. NADH augments blood flow in physiologically activated retina and visual cortex. Proc. Natl Acad. Sci. USA 101, 653–658 (2004)
Vlassenko, A. G., Rundle, M. M., Raichle, M. E. & Mintun, M. A. Regulation of blood flow in activated human brain by cytosolic NADH/NAD+ ratio. Proc. Natl Acad. Sci. USA 103, 1964–1969 (2006)
Kasischke, K. A., Vishwasrao, H. D., Fisher, P. J., Zipfel, W. R. & Webb, W. W. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 305, 99–103 (2004)
Vanzetta, I. & Grinvald, A. Increased cortical oxidative metabolism due to sensory stimulation: implications for functional brain imaging. Science 286, 1555–1558 (1999)
Ances, B. M., Buerk, D. G., Greenberg, J. H. & Detre, J. A. Temporal dynamics of the partial pressure of brain tissue oxygen during functional forepaw stimulation in rats. Neurosci. Lett. 306, 106–110 (2001)
Offenhauser, N., Thomsen, K., Caesar, K. & Lauritzen, M. Activity-induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow. J. Physiol. 565, 279–294 (2005)
Malonek, D. et al. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc. Natl Acad. Sci. USA 94, 14826–14831 (1997)
Devor, A. et al. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc. Natl Acad. Sci. USA 102, 3822–3827 (2005)
Fox, P. T. & Raichle, M. E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl Acad. Sci. USA 83, 1140–1144 (1986)
Fox, P. T., Raichle, M. E., Mintun, M. A. & Dence, C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 241, 462–464 (1988)
Hu, Y. & Wilson, G. S. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J. Neurochem. 69, 1484–1490 (1997)
Pellerin, L. & Magistretti, P. J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl Acad. Sci. USA 91, 10625–10629 (1994)
Hein, T. W., Xu, W. & Kuo, L. Dilation of retinal arterioles in response to lactate: role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Invest. Ophthalmol. Vis. Sci. 47, 693–699 (2006)
Yamanishi, S., Katsumura, K., Kobayashi, T. & Puro, D. G. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am. J. Physiol. Heart Circ. Physiol. 290, H925–H934 (2006)
Devor, A. et al. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J. Neurosci. 27, 4452–4459 (2007)
Ellis-Davies, G. C. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nature Methods 4, 619–628 (2007)
Chan, B. S., Endo, S., Kanai, N. & Schuster, V. L. Identification of lactate as a driving force for prostanoid transport by prostaglandin transporter PGT. Am. J. Physiol. Renal Physiol. 282, F1097–F1102 (2002)
Wender, R. et al. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J. Neurosci. 20, 6804–6810 (2000)
Chance, B., Cohen, P., Jobsis, F. & Schoener, B. Intracellular oxidation-reduction states in vivo. Science 137, 499–508 (1962)
Nimmerjahn, A., Kirchhoff, F., Kerr, J. N. & Helmchen, F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nature Methods 1, 31–37 (2004)
Frenguelli, B. G., Llaudet, E. & Dale, N. High-resolution real-time recording with microelectrode biosensors reveals novel aspects of adenosine release during hypoxia in rat hippocampal slices. J. Neurochem. 86, 1506–1515 (2003)
Brust, T. B., Cayabyab, F. S., Zhou, N. & MacVicar, B. A. p38 mitogen-activated protein kinase contributes to adenosine A1 receptor-mediated synaptic depression in area CA1 of the rat hippocampus. J. Neurosci. 26, 12427–12438 (2006)
Murphy, K. et al. Adenosine-A2a receptor down-regulates cerebral smooth muscle L-type Ca2+ channel activity via protein tyrosine phosphatase, not cAMP-dependent protein kinase. Mol. Pharmacol. 64, 640–649 (2003)
Chi, Y., Khersonsky, S. M., Chang, Y. T. & Schuster, V. L. Identification of a new class of prostaglandin transporter inhibitors and characterization of their biological effects on prostaglandin E2 transport. J. Pharmacol. Exp. Ther. 316, 1346–1350 (2006)
Chan, B. S., Satriano, J. A., Pucci, M. & Schuster, V. L. Mechanism of prostaglandin E2 transport across the plasma membrane of HeLa cells and Xenopus oocytes expressing the prostaglandin transporter ‘PGT’. J. Biol. Chem. 273, 6689–6697 (1998)
Fox, P. T. & Raichle, M. E. Stimulus rate dependence of regional cerebral blood flow in human striate cortex, demonstrated by positron emission tomography. J. Neurophysiol. 51, 1109–1120 (1984)
Kleinfeld, D., Mitra, P. P., Helmchen, F. & Denk, W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc. Natl Acad. Sci. USA 95, 15741–15746 (1998)
Chaigneau, E. et al. The relationship between blood flow and neuronal activity in the rodent olfactory bulb. J. Neurosci. 27, 6452–6460 (2007)
Cauli, B. et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J. Neurosci. 24, 8940–8949 (2004)
Peppiatt, C. M., Howarth, C., Mobbs, P. & Attwell, D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443, 700–704 (2006)
D’Agostino, D. P., Putnam, R. W. & Dean, J. B. Superoxide (·O2-) production in CA1 neurons of rat hippocampal slices exposed to graded levels of oxygen. J. Neurophysiol. 98, 1030–1041 (2007)
Xu, C., Zipfel, W., Shear, J. B., Williams, R. M. & Webb, W. W. Multiphoton fluorescence excitation: new spectral windows for biological nonlinear microscopy. Proc. Natl Acad. Sci. USA 93, 10763–10768 (1996)
Denk, W. Two-photon scanning photochemical microscopy: mapping ligand-gated ion channel distributions. Proc. Natl Acad. Sci. USA 91, 6629–6633 (1994)
Klaidman, L. K., Leung, A. C. & Adams, J. D. High-performance liquid chromatography analysis of oxidized and reduced pyridine dinucleotides in specific brain regions. Anal. Biochem. 228, 312–317 (1995)
Vishwasrao, H. D., Heikal, A. A., Kasischke, K. A. & Webb, W. W. Conformational dependence of intracellular NADH on metabolic state revealed by associated fluorescence anisotropy. J. Biol. Chem. 280, 25119–25126 (2005)
Sorg, O. & Magistretti, P. J. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res. 563, 227–233 (1991)
Brown, A. M. & Ransom, B. R. Astrocyte glycogen and brain energy metabolism. Glia 55, 1263–1271 (2007)
Ryu, J. K. et al. Microglial activation and cell death induced by the mitochondrial toxin 3-nitropropionic acid: in vitro and in vivo studies. Neurobiol. Dis. 12, 121–132 (2003)
Acknowledgements
We thank T. Murphy, T. Phillips and Y. Tian Wang for reading an earlier version of the manuscript. T34 was a gift from V. L. Schuster and Y. Chi. This work was supported by an operating grant from the Canadian Institutes of Health Research. B.A.M. is a Canada Research Chair and a Michael Smith Foundation for Health Research (MSFHR) Distinguished Scholar. G.R.J.G. is supported by fellowships from the Alberta Heritage Foundation for Medical Research, MSFHR and the Natural Science and Engineering Council of Canada. H.B.C. is supported by postdoctoral fellowships from Wilms Foundation and the Heart and Stroke Foundation of Canada.
Author Contributions G.R.J.G. and B.A.M. designed the imaging experiments and wrote the manuscript. G.R.J.G. performed the imaging experiments and analysis, took slice pO2 measurements and tested the effects of synaptic activation on vasomotion. H.B.C., G.R.J.G. and B.A.M. designed the lactate and PGE2 experiments. H.B.C. and B.A.M. designed the immunohistochemistry experiments. H.B.C. performed the lactate and PGE2 measurements and analysis and the immunohistochemistry. R.L.R. performed the extracellular field recordings examining adenosine tone. G.C.R.E.-D. designed and synthesized the calcium cage. All authors helped to edit the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-8 with Legends (PDF 6090 kb)
Rights and permissions
About this article
Cite this article
Gordon, G., Choi, H., Rungta, R. et al. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456, 745–749 (2008). https://doi.org/10.1038/nature07525
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07525
This article is cited by
-
Aquaporin-4 Deficiency is Associated with Cognitive Impairment and Alterations in astrocyte-neuron Lactate Shuttle
Molecular Neurobiology (2023)
-
The Memory Orchestra: Contribution of Astrocytes
Neuroscience Bulletin (2023)
-
Hypertonic lactate for the treatment of intracranial hypertension in patients with acute brain injury
Scientific Reports (2022)
-
Heterogeneity of Astrocytes in Grey and White Matter
Neurochemical Research (2021)
-
Occupation-related effects on motor cortex thickness among older, cognitive healthy individuals
Brain Structure and Function (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.