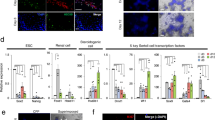
Abstract
Human primordial germ cells and mouse neonatal and adult germline stem cells are pluripotent and show similar properties to embryonic stem cells. Here we report the successful establishment of human adult germline stem cells derived from spermatogonial cells of adult human testis. Cellular and molecular characterization of these cells revealed many similarities to human embryonic stem cells, and the germline stem cells produced teratomas after transplantation into immunodeficient mice. The human adult germline stem cells differentiated into various types of somatic cells of all three germ layers when grown under conditions used to induce the differentiation of human embryonic stem cells. We conclude that the generation of human adult germline stem cells from testicular biopsies may provide simple and non-controversial access to individual cell-based therapy without the ethical and immunological problems associated with human embryonic stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
30 July 2014
A Correction to this paper has been published: https://doi.org/10.1038/nature13661
References
de Rooij, D. G. Rapid expansion of the spermatogonial stem cell tool box. Proc. Natl Acad. Sci. USA 103, 7939–7940 (2006)
Brinster, R. L. & Avarbock, M. R. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl Acad. Sci. USA 91, 11303–11307 (1994)
Kubota, H. & Brinster, R. L. Technology insight: In vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nature Clin. Pract. Endocrinol. Metab. 2, 99–108 (2006)
Matsui, Y., Zsebo, K. & Hogan, B. L. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell 70, 841–847 (1992)
Kanatsu-Shinohara, M. et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell 119, 1001–1012 (2004)
Guan, K. et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 440, 1199–1203 (2006)
Stevens, L. C. Spontaneous and experimentally induced testicular teratomas in mice. Cell Differ. 15, 69–74 (1984)
Resnick, J. L., Bixler, L. S., Cheng, L. & Donovan, P. J. Long-term proliferation of mouse primordial germ cells in culture. Nature 359, 550–551 (1992)
Turnpenny, L. et al. Evaluating human embryonic germ cells: concord and conflict as pluripotent stem cells. Stem Cells 24, 212–220 (2006)
Seandel, M. et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature 449, 346–350 (2007)
Meng, X. et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287, 1489–1493 (2000)
Kanatsu-Shinohara, M. et al. Leukemia inhibitory factor enhances formation of germ cell colonies in neonatal mouse testis culture. Biol. Reprod. 76, 55–62 (2007)
Shamblott, M. J. et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl Acad. Sci. USA 95, 13726–13731 (1998)
Shamblott, M. J. et al. Human embryonic germ cell derivatives express a broad range of developmentally distinct markers and proliferate extensively in vitro . Proc. Natl Acad. Sci. USA 98, 113–118 (2001)
Costoya, J. A. et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nature Genet. 36, 653–659 (2004)
Shinohara, T., Avarbock, M. R. & Brinster, R. L. β1- and α6-integrin are surface markers on mouse spermatogonial stem cells. Proc. Natl Acad. Sci. USA 96, 5504–5509 (1999)
Kubota, H., Avarbock, M. R. & Brinster, R. L. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol. Reprod. 71, 722–731 (2004)
Stukenborg, J. B. et al. Co-culture of spermatogonia with somatic cells in a novel three-dimensional soft-agar-culture-system. J. Androl. 29, 312–329 (2007)
Hamra, F. K. et al. Defining the spermatogonial stem cell. Dev. Biol. 269, 393–410 (2004)
Wernig, M. et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324 (2007)
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007)
Park, I. H. et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451, 141–146 (2008)
Meissner, A., Wernig, M. & Jaenisch, R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nature Biotechnol. 25, 1177–1181 (2007)
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007)
Ginis, I. et al. Differences between human and mouse embryonic stem cells. Dev. Biol. 269, 360–380 (2004)
Schatten, G., Smith, J., Navara, C., Park, J. H. & Pedersen, R. Culture of human embryonic stem cells. Nature Methods 2, 455–463 (2005)
Maltsev, V. A., Rohwedel, J., Hescheler, J. & Wobus, A. M. Embryonic stem cells differentiate in vitro into cardiomyocytes representing sinusnodal, atrial and ventricular cell types. Mech. Dev. 44, 41–50 (1993)
Kehat, I. et al. Development of cardiomyocytes from human ES cells. Methods Enzymol. 365, 461–473 (2003)
Ringe, J., Haupl, T. & Sittinger, M. Mesenchymal stem cells for tissue engineering of bone and cartilage. Med. Klin. 98 (suppl. 2). 35–40 (2003)
Bielby, R. C., Boccaccini, A. R., Polak, J. M. & Buttery, L. D. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 10, 1518–1525 (2004)
Lumelsky, N. et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 292, 1389–1394 (2001)
Segev, H., Fishman, B., Ziskind, A., Shulman, M. & Itskovitz-Eldor, J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells 22, 265–274 (2004)
Blyszczuk, P. et al. Embryonic stem cells differentiate into insulin-producing cells without selection of nestin-expressing cells. Int. J. Dev. Biol. 48, 1095–1104 (2004)
Pollard, S. M., Conti, L., Sun, Y., Goffredo, D. & Smith, A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb. Cortex 16 (suppl. 1). i112–i120 (2006)
Bibel, M. et al. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nature Neurosci. 7, 1003–1009 (2004)
Wittwer, C. T., Fillmore, G. C. & Hillyard, D. R. Automated polymerase chain reaction in capillary tubes with hot air. Nucleic Acids Res. 17, 4353–4357 (1989)
Alexander, D. et al. Transcription factor Egr-1 activates collagen expression in immortalized fibroblasts or fibrosarcoma cells. Biol. Chem. 383, 1845–1853 (2002)
Acknowledgements
We thank S. Singer, U. Mau-Holzmann and O. Rieß for karyotyping the human adult GSCs obtained from the patients’ biopsies; M. Schenk for providing support with ELISA; D. Blaurock for proofreading the manuscript; K. Kohler for discussion and encouragement; and P. Bauer for conducting the microsatellite analysis. Financial support for this research was provided by the Department of Urology, Institute of Anatomy and ZRM (University Clinic Tübingen).
Author Contributions S.C. conducted all the major experiments and wrote the manuscript; M.R. and Jö.H. were responsible for the clinical logistics, organization of the human testis biopsies and writing the ethical proposal; L.J. supervised the experimental procedures conducted in Tübingen; M.R., M.M. and Jü.H. provided mRNA and cDNA from human ES cells (H1), determined the doubling time of H1 cells and were involved in the differentiation experiments for comparing H1 with human adult GSCs; T.W. conducted part of the PCR experiments; W.A. was responsible for the real-time PCR experiments, M.B. was responsible for microarray profiling, H.-J.B. was co-responsible for FACS analysis; U.M. and H.-J.W. conducted the electron microscopy study; A.M. performed confocal microscopy; S.M. provided human ES cells; K.-D.S. and A.S. supported and advised the clinical cooperation; and T.S. initiated and supervised the entire project, conducted parts of the experiments and wrote the manuscript. A.S. and T.S. are co-senior authors.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9 with Legends and Supplementary Tables 1-5. This file was revised on 23 October 2008 to incorporate a correction to Supplementary Figure 1 (the diagram next to the right mouse icon should read ‘teratoma fomation’ and not ‘no teratoma fomation’ as previously shown). (PDF 9498 kb)
Rights and permissions
About this article
Cite this article
Conrad, S., Renninger, M., Hennenlotter, J. et al. Generation of pluripotent stem cells from adult human testis. Nature 456, 344–349 (2008). https://doi.org/10.1038/nature07404
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07404
This article is cited by
-
Learning Towards Maturation of Defined Feeder-free Pluripotency Culture Systems: Lessons from Conventional Feeder-based Systems
Stem Cell Reviews and Reports (2024)
-
Current Progress in Stem Cell Therapy for Male Infertility
Stem Cell Reviews and Reports (2023)
-
Kardiale Zelltherapie – „lost in translation?“
Zeitschrift für Herz-,Thorax- und Gefäßchirurgie (2022)
-
Differential effects of extracellular matrix proteins on in vitro culture and growth characteristics of caprine male germ cells
In Vitro Cellular & Developmental Biology - Animal (2021)
-
Regenerative Medicine Approaches in Bioengineering Female Reproductive Tissues
Reproductive Sciences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.