Abstract
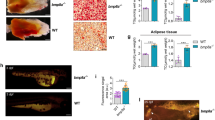
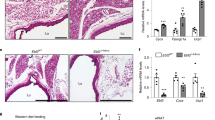
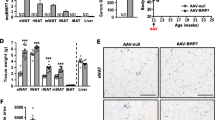
Adipose tissue is central to the regulation of energy balance. Two functionally different types of fat are present in mammals: white adipose tissue, the primary site of triglyceride storage, and brown adipose tissue, which is specialized in energy expenditure and can counteract obesity1. Factors that specify the developmental fate and function of white and brown adipose tissue remain poorly understood2,3. Here we demonstrate that whereas some members of the family of bone morphogenetic proteins (BMPs) support white adipocyte differentiation, BMP7 singularly promotes differentiation of brown preadipocytes even in the absence of the normally required hormonal induction cocktail. BMP7 activates a full program of brown adipogenesis including induction of early regulators of brown fat fate PRDM16 (PR-domain-containing 16; ref. 4) and PGC-1α (peroxisome proliferator-activated receptor-γ (PPARγ) coactivator-1α; ref. 5), increased expression of the brown-fat-defining marker uncoupling protein 1 (UCP1) and adipogenic transcription factors PPARγ and CCAAT/enhancer-binding proteins (C/EBPs), and induction of mitochondrial biogenesis via p38 mitogen-activated protein (MAP) kinase-(also known as Mapk14) and PGC-1-dependent pathways. Moreover, BMP7 triggers commitment of mesenchymal progenitor cells to a brown adipocyte lineage, and implantation of these cells into nude mice results in development of adipose tissue containing mostly brown adipocytes. Bmp7 knockout embryos show a marked paucity of brown fat and an almost complete absence of UCP1. Adenoviral-mediated expression of BMP7 in mice results in a significant increase in brown, but not white, fat mass and leads to an increase in energy expenditure and a reduction in weight gain. These data reveal an important role of BMP7 in promoting brown adipocyte differentiation and thermogenesis in vivo and in vitro, and provide a potential new therapeutic approach for the treatment of obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gesta, S., Tseng, Y. H. & Kahn, C. R. Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256 (2007)
Farmer, S. R. Transcriptional control of adipocyte formation. Cell Metab. 4, 263–273 (2006)
Rosen, E. D. & MacDougald, O. A. Adipocyte differentiation from the inside out. Nature Rev. Mol. Cell Biol. 7, 885–896 (2006)
Seale, P. et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6, 38–54 (2007)
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998)
Chen, D., Zhao, M. & Mundy, G. R. Bone morphogenetic proteins. Growth Factors 22, 233–241 (2004)
Tseng, Y. H. & He, T. C. Bone morphogenetic proteins and adipocyte differentiation. Cellscience Rev. 3, 342–360 (2007)
Wang, E. A., Israel, D. I., Kelly, S. & Luxenberg, D. P. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors 9, 57–71 (1993)
Tang, Q. Q., Otto, T. C. & Lane, M. D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl Acad. Sci. USA 101, 9607–9611 (2004)
Klein, J. et al. β3-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin induced glucose uptake in brown adipocytes. J. Biol. Chem. 274, 34795–34802 (1999)
Ducy, P., Zhang, R., Geoffroy, V., Ridall, A. L. & Karsenty, G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747–754 (1997)
Canalis, E., Economides, A. N. & Gazzerro, E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 24, 218–235 (2003)
Puigserver, P. et al. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARγ coactivator-1. Mol. Cell 8, 971–982 (2001)
Cao, W. et al. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 24, 3057–3067 (2004)
Uldry, M. et al. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 3, 333–341 (2006)
Tseng, Y. H. et al. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nature Cell Biol. 7, 601–611 (2005)
Nedergaard, J., Connally, E. & Cannon, B. Brown Adipose Tissue (eds Trayhurn, P. & Nicholls, D. G.) 152–213 (Arnold, 1986)
Varga, A. C. & Wrana, J. L. The disparate role of BMP in stem cell biology. Oncogene 24, 5713–5721 (2005)
Darlington, G. J., Ross, S. E. & MacDougald, O. A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 273, 30057–30060 (1998)
Wu, Z. et al. Cross-regulation of C/EBPαand PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 3, 151–158 (1999)
Tseng, Y. H., Kriauciunas, K. M., Kokkotou, E. & Kahn, C. R. Differential roles of insulin receptor substrates in brown adipocyte differentiation. Mol. Cell. Biol. 24, 1918–1929 (2004)
Dudley, A. T., Lyons, K. M. & Robertson, E. J. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 9, 2795–2807 (1995)
Luo, G. et al. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 9, 2808–2820 (1995)
Jin, W. et al. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev. Cell 10, 461–471 (2006)
Cheng, H. et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J. Bone Joint Surg. Am. 85-A, 1544–1552 (2003)
Zeisberg, M. et al. BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nature Med. 9, 964–968 (2003)
Ozkaynak, E. et al. OP-1 cDNA encodes an osteogenic protein in the TGF-β family. EMBO J. 9, 2085–2093 (1990)
Tobin, J. F. & Celeste, A. J. Bone morphogenetic proteins and growth differentiation factors as drug targets in cardiovascular and metabolic disease. Drug Discov. Today 11, 405–411 (2006)
Fasshauer, M. et al. Essential role of insulin receptor substrate-2 in insulin stimulation of glut4 translocation and glucose uptake in brown adipocytes. J. Biol. Chem. 275, 25494–25501 (2000)
Fasshauer, M. et al. Essential role of insulin receptor substrate 1 in differentiation of brown adipocytes. Mol. Cell. Biol. 21, 319–329 (2001)
Tseng, Y. H., Ueki, K., Kriauciunas, K. M. & Kahn, C. R. Differential roles of insulin receptor substrates in the anti-apoptotic function of insulin-like growth factor-1 and insulin. J. Biol. Chem. 277, 31601–31611 (2002)
Hauner, H. et al. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J. Clin. Invest. 84, 1663–1670 (1989)
Godin, R. E., Takaesu, N. T., Robertson, E. J. & Dudley, A. T. Regulation of BMP7 expression during kidney development. Development 125, 3473–3482 (1998)
Bandyopadhyay, A. et al. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2, e216 (2006)
Ueki, K., Kondo, T., Tseng, Y. H. & Kahn, C. R. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc. Natl Acad. Sci. USA 101, 10422–10427 (2004)
Laustsen, P. G. et al. Lipoatrophic diabetes in Irs1-/-/Irs3-/- double knockout mice. Genes Dev. 16, 3213–3222 (2002)
Acknowledgements
We acknowledge T.-C. He for providing adenoviruses expressing BMPs. We thank M. Rourk and L. Mazzola for help with the animal experiments, J. Hu, C. Cahill, A. McSweeney, L. Polivy and R. Bronson for technical assistance, and P. Zhang for statistical consultation. We thank A. Butte and P. Laustsen for inputs on initiation of this project. We thank M. Uldry and B. Spiegelman for providing the PGC-1 null cells. This work was supported in part by the National Institutes of Health grants R01 DK077097, R21 DK070722, P30 DK46200 and P30 DK040561 (to Y.-H.T.), R01 DK67536 (to R.N.K.), K08 DK64906 (to A.W.N.) and R01 DK 060837 (to C.R.K), the Tanita Healthy Weight Community, and the Eleanor and Miles Shore 50th Anniversary Scholar Program from Harvard Medical School (to Y.-H.T.).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures S1-S14 and Supplementary Tables S1-S2. (PDF 637 kb)
Rights and permissions
About this article
Cite this article
Tseng, YH., Kokkotou, E., Schulz, T. et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454, 1000–1004 (2008). https://doi.org/10.1038/nature07221
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07221
This article is cited by
-
Adipogenic effects of Ostreae Testa water extract on white adipocytes
Molecular & Cellular Toxicology (2024)
-
Huangqi decoction ameliorates kidney injury in db/db mice by regulating the BMP/Smad signaling pathway
BMC Complementary Medicine and Therapies (2023)
-
Bmp8a deletion leads to obesity through regulation of lipid metabolism and adipocyte differentiation
Communications Biology (2023)
-
An update on the secretory functions of brown, white, and beige adipose tissue: Towards therapeutic applications
Reviews in Endocrine and Metabolic Disorders (2023)
-
Omentin-1 drives cardiomyocyte cell cycle arrest and metabolic maturation by interacting with BMP7
Cellular and Molecular Life Sciences (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.