Abstract
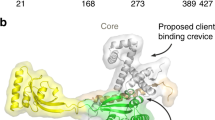
All organisms have to monitor the folding state of cellular proteins precisely. The heat-shock protein DegP is a protein quality control factor in the bacterial envelope that is involved in eliminating misfolded proteins and in the biogenesis of outer-membrane proteins. Here we describe the molecular mechanisms underlying the regulated protease and chaperone function of DegP from Escherichia coli. We show that binding of misfolded proteins transforms hexameric DegP into large, catalytically active 12-meric and 24-meric multimers. A structural analysis of these particles revealed that DegP represents a protein packaging device whose central compartment is adaptable to the size and concentration of substrate. Moreover, the inner cavity serves antagonistic functions. Whereas the encapsulation of folded protomers of outer-membrane proteins is protective and might allow safe transit through the periplasm, misfolded proteins are eliminated in the molecular reaction chamber. Oligomer reassembly and concomitant activation on substrate binding may also be critical in regulating other HtrA proteases implicated in protein-folding diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Primary accessions
Protein Data Bank
Data deposits
The DegP24 structure is deposited in the Protein Data Bank under accession number 3cs0. The fitted model of the electron microscopic three-dimensional map of DegP12–OMP is deposited in the Protein Data Bank under accession number 2zle. The electron-microscopic three-dimensional maps are deposited at the Electron Microscopy Data Bank (http://www.ebi.ac.uk/msd-srv/emsearch/index.html) under accession codes EMD-1504 and EMD-1505 for DegP24–OMP and DegP12–OMP, respectively.
References
Selkoe, D. J. Folding proteins in fatal ways. Nature 426, 900–904 (2003)
Macario, A. J. & Conway de Macario, E. Sick chaperones, cellular stress, and disease. N. Engl. J. Med. 353, 1489–1501 (2005)
Spiess, C., Beil, A. & Ehrmann, M. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97, 339–347 (1999)
Iwanczyk, J. et al. Role of the PDZ domains in Escherichia coli DegP protein. J. Bacteriol. 189, 3176–3186 (2007)
Meltzer, M. et al. Allosteric activation of HtrA protease DegP by stress signals during bacterial protein quality control. Angew. Chem. Int. Edn Engl. 47, 1332–1334 (2008)
Clausen, T., Southan, C. & Ehrmann, M. The HtrA family of proteases: implications for protein composition and cell fate. Mol. Cell 10, 443–455 (2002)
Grau, S. et al. Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl Acad. Sci. USA 102, 6021–6026 (2005)
Plun-Favreau, H. et al. The mitochondrial protease HtrA2 is regulated by Parkinson’s disease-associated kinase PINK1. Nature Cell Biol. 9, 1243–1252 (2007)
Harris, B. Z. & Lim, W. A. Mechanism and role of PDZ domains in signaling complex assembly. J. Cell Sci. 114, 3219–3231 (2001)
Wilken, C., Kitzing, K., Kurzbauer, R., Ehrmann, M. & Clausen, T. Crystal structure of the DegS stress sensor: How a PDZ domain recognizes misfolded protein and activates a protease. Cell 117, 483–494 (2004)
Hasselblatt, H. et al. Regulation of the σE stress response by DegS: how the PDZ domain keeps the protease inactive in the resting state and allows integration of different OMP-derived stress signals upon folding stress. Genes Dev. 21, 2659–2670 (2007)
Krojer, T., Garrido-Franco, M., Huber, R., Ehrmann, M. & Clausen, T. Crystal structure of DegP (HtrA) reveals a new protease-chaperone machine. Nature 416, 455–459 (2002)
Misra, R., Peterson, A., Ferenci, T. & Silhavy, T. J. A genetic approach for analyzing the pathway of LamB assembly into the outer membrane of Escherichia coli . J. Biol. Chem. 266, 13592–13597 (1991)
Misra, R., CastilloKeller, M. & Deng, M. Overexpression of protease-deficient DegP(S210A) rescues the lethal phenotype of Escherichia coli OmpF assembly mutants in a degP background. J. Bacteriol. 182, 4882–4888 (2000)
CastilloKeller, M. & Misra, R. Protease-deficient DegP suppresses lethal effects of a mutant OmpC protein by its capture. J. Bacteriol. 185, 148–154 (2003)
Purdy, G. E., Fisher, C. R. & Payne, S. M. IcsA surface presentation in Shigella flexneri requires the periplasmic chaperones DegP, Skp, and SurA. J. Bacteriol. 189, 5566–5573 (2007)
Ruiz, N., Kahne, D. & Silhavy, T. J. Advances in understanding bacterial outer-membrane biogenesis. Nat. Rev. Microbiol. 4, 57–66 (2006)
Wu, T. et al. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli . Cell 121, 235–245 (2005)
Alba, B. M. & Gross, C. A. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol. Microbiol. 52, 613–619 (2004)
Chen, R. & Henning, U. A periplasmic protein (Skp) of Escherichia coli selectively binds a class of outer membrane proteins. Mol. Microbiol. 19, 1287–1294 (1996)
Rouviere, P. E. & Gross, C. A. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev. 10, 3170–3182 (1996)
Rizzitello, A. E., Harper, J. R. & Silhavy, T. J. Genetic evidence for parallel pathways of chaperone activity in the periplasm of Escherichia coli . J. Bacteriol. 183, 6794–6800 (2001)
Sklar, J. G., Wu, T., Kahne, D. & Silhavy, T. J. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli . Genes Dev. 21, 2473–2484 (2007)
Rhodius, V. A., Suh, W. C., Nonaka, G., West, J. & Gross, C. A. Conserved and variable functions of the σE stress response in related genomes. PLoS Biol. 4, e2 (2006)
Guisbert, E., Rhodius, V. A., Ahuja, N., Witkin, E. & Gross, C. A. Hfq modulates the σE-mediated envelope stress response and the σ32-mediated cytoplasmic stress response in Escherichia coli . J. Bacteriol. 189, 1963–1973 (2007)
Schweizer, M., Hindennach, I., Garten, W. & Henning, U. Major proteins of the Escherichia coli outer cell envelope membrane. Interaction of protein II with lipopolysaccharide. Eur. J. Biochem. 82, 211–217 (1978)
Sen, K. & Nikaido, H. Lipopolysaccharide structure required for in vitro trimerization of Escherichia coli OmpF porin. J. Bacteriol. 173, 926–928 (1991)
Zimmermann, P. et al. PIP(2)-PDZ domain binding controls the association of syntenin with the plasma membrane. Mol. Cell 9, 1215–1225 (2002)
Yan, J. et al. Structure of the split PH domain and distinct lipid-binding properties of the PH-PDZ supramodule of α-syntrophin. EMBO J. 24, 3985–3995 (2005)
Mortier, E. et al. Nuclear speckles and nucleoli targeting by PIP2–PDZ domain interactions. EMBO J. 24, 2556–2565 (2005)
Pan, L. et al. Clustering and synaptic targeting of PICK1 requires direct interaction between the PDZ domain and lipid membranes. EMBO J. 26, 4576–4587 (2007)
Wu, H. et al. PDZ domains of Par-3 as potential phosphoinositide signaling integrators. Mol. Cell 28, 886–898 (2007)
Dubochet, J., McDowall, A. W., Menge, B., Schmid, E. N. & Lickfeld, K. G. Electron microscopy of frozen-hydrated bacteria. J. Bacteriol. 155, 381–390 (1983)
Leduc, M., Frehel, C. & van Heijenoort, J. Correlation between degradation and ultrastructure of peptidoglycan during autolysis of Escherichia coli . J. Bacteriol. 161, 627–635 (1985)
Winkler, F. K., Schutt, C. E., Harrison, S. C. & Bricogne, G. Tomato bushy stunt virus at 5.5-Å resolution. Nature 265, 509–513 (1977)
Banyard, S. H., Stammers, D. K. & Harrison, P. M. Electron density map of apoferritin at 2.8-Å resolution. Nature 271, 282–284 (1978)
Park, S., Liu, G., Topping, T. B., Cover, W. H. & Randall, L. L. Modulation of folding pathways of exported proteins by the leader sequence. Science 239, 1033–1035 (1988)
de Cock, H., Hendriks, R., de Vrije, T. & Tommassen, J. Assembly of an in vitro synthesized Escherichia coli outer membrane porin into its stable trimeric configuration. J. Biol. Chem. 265, 4646–4651 (1990)
Klose, M. et al. Membrane assembly of the outer membrane protein OmpA of Escherichia coli . J. Biol. Chem. 268, 25664–25670 (1993)
Rouviere, P. E. & Gross, C. A. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev. 10, 3170–3182 (1996)
Perona, J. J. & Craik, C. S. Structural basis of substrate specificity in the serine proteases. Protein Sci. 4, 337–360 (1995)
DeLano, W. L. The PyMOL Molecular Graphics System (DeLano Scientific, Palo Alto, CA, 2002)
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Cryst. 26, 795–800 (1993)
Weeks, C. M. & Miller, R. The design and implementation of SnB version 2.0. J. Appl. Cryst. 32, 120–124 (1999)
de la Fortelle, E. & Bricogne, G. Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol. 276, 472–494 (1997)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron-density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Brunger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Ludtke, S. J., Baldwin, P. R. & Chiu, W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 128, 82–97 (1999)
Frank, J. et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 116, 190–199 (1996)
van Heel, M., Harauz, G., Orlova, E. V., Schmidt, R. & Schatz, M. A new generation of the IMAGIC image processing system. J. Struct. Biol. 116, 17–24 (1996)
Penczek, P., Radermacher, M. & Frank, J. Three-dimensional reconstruction of single particles embedded in ice. Ultramicroscopy 40, 33–53 (1992)
van Heel, M. Angular reconstitution: a posteriori assignment of projection directions for 3D reconstruction. Ultramicroscopy 21, 111–123 (1987)
Basle, A., Rummel, G., Storici, P., Rosenbusch, J. P. & Schirmer, T. Crystal structure of osmoporin OmpC from E. coli at 2.0 Å. J. Mol. Biol. 362, 933–942 (2006)
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004)
Matsuyama, S., Inokuchi, K. & Mizushima, S. Promoter exchange between Ompf and Ompc, genes for osmoregulated major outer-membrane proteins of Escherichia coli K-12. J. Bacteriol. 158, 1041–1047 (1984)
Nakae, T., Ishii, J. & Tokunaga, M. Subunit structure of functional porin oligomers that form permeability channels in the outer membrane of Escherichia coli . J. Biol. Chem. 254, 1457–1461 (1979)
Acknowledgements
We thank K. Mechtler and I. Steinmacher for assistance with mass spectrometry; the staff at the European Synchrotron Radiation Facility and the Swiss Light Source for assistance with collecting synchrotron data; N. Boisset and R. Trujillo for providing image-processing scripts; L. Wang for support with electron microscopy; D. Houldershaw for computer support; and E. Orlova for discussion. The Research Institute of Molecular Pathology (IMP) is funded by Boehringer Ingelheim. E.S. and H.R.S. were supported by the UK Biotechnology and Biological Sciences Research Council, M.E. by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie, T.C. by the EMBO Young Investigator Program, and T.K. and J.S. by the Austrian Science Fund.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
The file contains Supplementary Table 1 and Supplementary Figures S1-S6 with Legends. The Table comprises a summary of X-ray data collection, phasing and refinement statistics. The Figures illustrate the identification and isolation of the DegP/OMP complex (S1), EM images of DegP12/OMP and DegP24/OMP complexes (S2), a structural comparison of the overall dimensions of DegP6 and DegP24 (S3), the intersubunit contacts of different DegP oligomers (S4), the biochemical analysis of oligomer formation and protease activity (S5) and the Fourier shell correlation curves of the EM maps (S6). (PDF 1152 kb)
Rights and permissions
About this article
Cite this article
Krojer, T., Sawa, J., Schäfer, E. et al. Structural basis for the regulated protease and chaperone function of DegP. Nature 453, 885–890 (2008). https://doi.org/10.1038/nature07004
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07004
This article is cited by
-
In-cell kinetic stability is an essential trait in metallo-β-lactamase evolution
Nature Chemical Biology (2023)
-
Role of DegQ in differential stability of flagellin subunits in Vibrio vulnificus
npj Biofilms and Microbiomes (2021)
-
A novel FRET peptide assay reveals efficient Helicobacter pylori HtrA inhibition through zinc and copper binding
Scientific Reports (2020)
-
FAM111A protects replication forks from protein obstacles via its trypsin-like domain
Nature Communications (2020)
-
Bam complex-mediated assembly of bacterial outer membrane proteins synthesized in an in vitro translation system
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.