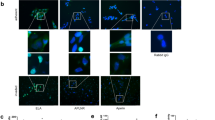
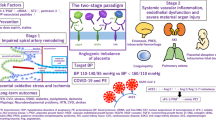
Abstract
Despite intense investigation, mechanisms that facilitate the emergence of the pre-eclampsia phenotype in women are still unknown. Placental hypoxia, hypertension, proteinuria and oedema are the principal clinical features of this disease. It is speculated that hypoxia-driven disruption of the angiogenic balance involving vascular endothelial growth factor (VEGF)/placenta-derived growth factor (PLGF) and soluble Fms-like tyrosine kinase-1 (sFLT-1, the soluble form of VEGF receptor 1) might contribute to some of the maternal symptoms of pre-eclampsia1,2,3,4,5. However, pre-eclampsia does not develop in all women with high sFLT-1 or low PLGF levels, and it also occurs in some women with low sFLT-1 and high PLGF levels5,6. Moreover, recent experiments strongly suggest that several soluble factors affecting the vasculature are probably elevated because of placental hypoxia in the pre-eclamptic women, indicating that upstream molecular defect(s) may contribute to pre-eclampsia. Here we show that pregnant mice deficient in catechol-O-methyltransferase (COMT) show a pre-eclampsia-like phenotype resulting from an absence of 2-methoxyoestradiol (2-ME), a natural metabolite of oestradiol that is elevated during the third trimester of normal human pregnancy. 2-ME ameliorates all pre-eclampsia-like features without toxicity in the Comt-/- pregnant mice and suppresses placental hypoxia, hypoxia-inducible factor-1α expression and sFLT-1 elevation. The levels of COMT and 2-ME are significantly lower in women with severe pre-eclampsia. Our studies identify a genetic mouse model for pre-eclampsia and suggest that 2-ME may have utility as a plasma and urine diagnostic marker for this disease, and may also serve as a therapeutic supplement to prevent or treat this disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vuorela, P. et al. Amniotic fluid–soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet. Gynecol. 95, 353–357 (2000)
Sugimoto, H. et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J. Biol. Chem. 278, 12605–12608 (2003)
Krauss, T., Pauer, H. U. & Augustin, H. G. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens. Pregnancy 23, 101–111 (2004)
Maynard, S. E. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 111, 649–658 (2003)
Levine, R. J. et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 350, 672–683 (2004)
Solomon, C. G. & Seely, E. W. Preeclampsia—searching for the cause. N. Engl. J. Med. 350, 641–642 (2004)
Casey, M. L. & MacDonald, P. C. Characterization of catechol-O-methyltransferase activity in human uterine decidua vera tissue. Am. J. Obstet. Gynecol. 145, 453–457 (1983)
Berg, D., Sonsalla, R. & Kuss, E. Concentrations of 2-methoxyoestrogens in human serum measured by a heterologous immunoassay with an 125I-labelled ligand. Acta Endocrinol. (Copenh.) 103, 282–288 (1983)
Fotsis, T. et al. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature 368, 237–239 (1994)
D'Amato, R. J., Lin, C. M., Flynn, E., Folkman, J. & Hamel, E. 2-Methoxyestradiol, an endogenous mammalian metabolite, inhibits tubulin polymerization by interacting at the colchicine site. Proc. Natl Acad. Sci. USA 91, 3964–3968 (1994)
Mabjeesh, N. J. et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 3, 363–375 (2003)
Semenza, G. L. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr. Opin. Genet. Dev. 8, 588–594 (1998)
Barnea, E. R., MacLusky, N. J., DeCherney, A. H. & Naftolin, F. Catechol-O-methyl transferase activity in the human term placenta. Am. J. Perinatol. 5, 121–127 (1988)
Senthilkumaran, B. & Joy, K. P. Periovulatory changes in catfish ovarian oestradiol-17β, oestrogen-2-hydroxylase and catechol-O-methyltransferase during GnRH analogue-induced ovulation and in vitro induction of oocyte maturation by catecholoestrogens. J. Endocrinol. 168, 239–247 (2001)
Ireson, C. R. et al. Pharmacokinetics and efficacy of 2-methoxyoestradiol and 2-methoxyoestradiol-bis-sulphamate in vivo in rodents. Br. J. Cancer 90, 932–937 (2004)
Zacharia, L. C. et al. 2-hydroxyestradiol is a prodrug of 2-methoxyestradiol. J. Pharmacol. Exp. Ther. 309, 1093–1097 (2004)
Redman, C. W. & Sargent, I. L. Latest advances in understanding preeclampsia. Science 308, 1592–1594 (2005)
Labarrere, C. A. Acute atherosis. A histopathological hallmark of immune aggression? Placenta 9, 95–108 (1988)
MacGillivray, I., Rose, G. A. & Rowe, B. Blood pressure survey in pregnancy. Clin. Sci. 37, 395–407 (1969)
Genbacev, O., Joslin, R., Damsky, C. H., Polliotti, B. M. & Fisher, S. J. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J. Clin. Invest. 97, 540–550 (1996)
Caniggia, I. et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ3 . J. Clin. Invest. 105, 577–587 (2000)
Nagamatsu, T. et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 145, 4838–4845 (2004)
Ahmad, S. & Ahmed, A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ. Res. 95, 884–891 (2004)
Myatt, L., Eis, A. L., Brockman, D. E., Greer, I. A. & Lyall, F. Endothelial nitric oxide synthase in placental villous tissue from normal, pre-eclamptic and intrauterine growth restricted pregnancies. Hum. Reprod. 12, 167–172 (1997)
Hata, T., Miyazaki, K. & Matsui, K. Decreased circulating adrenomedullin in pre-eclampsia. Lancet 350, 1600–1605 (1997)
Rajakumar, A., Brandon, H. M., Daftary, A., Ness, R. & Conrad, K. P. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 25, 763–769 (2004)
Takanashi, K., Honma, T., Kashiwagi, T., Honjo, H. & Yoshizawa, I. Detection and measurement of urinary 2-hydroxyestradiol 17-sulfate, a potential placental antioxidant during pregnancy. Clin. Chem. 46, 373–378 (2000)
Rosing, U. & Carlstrom, K. Serum levels of unconjugated and total oestrogens and dehydroepiandrosterone, progesterone and urinary oestriol excretion in pre-eclampsia. Gynecol. Obstet. Invest. 18, 199–205 (1984)
Sata, F. et al. Functional maternal catechol-O-methyltransferase polymorphism and fetal growth restriction. Pharmacogenet. Genomics 16, 775–781 (2006)
Gogos, J. A. et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc. Natl Acad. Sci. USA 95, 9991–9996 (1998)
Sugimoto, H. et al. Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc. Natl Acad. Sci. USA 103, 7321–7326 (2006)
Walzer, T. et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc. Natl Acad. Sci. USA 104, 3384–3389 (2007)
Tanaka, T. S. et al. Genome-wide expression profiling of mid-gestation placenta and embryo using a 15,000 mouse developmental cDNA microarray. Proc. Natl Acad. Sci. USA 97, 9127–9132 (2000)
Acknowledgements
We dedicate this manuscript to Judah Folkman for his inspiration and guidance. We thank P. T. Männistö for providing us with information about the Comt-/- mice. Beth Israel Deaconess Medical Center has licensed technologies associated with 2-ME/COMT and preeclampsia to Cynthus, Inc. This work was supported primarily by the BIDMC Department of Medicine research funds to the Division of Matrix Biology, and partly supported by National Institutes of Health grants DK 55001, DK 62987, DK 13193 and DK 61688. S.A. was supported by grants from the British Heart Foundation and the Medical Research Council. K.K. was partly supported by Foreign Study Grants from the Kanae Foundation for the Promotion of Medical Science in Japan.
Author Contributions K.K. performed all the experiments, analysed the data and participated in manuscript writing. K.P., H.S., Y.H. and L.X. performed some of the experiments. V.H.G. performed the EM analysis. S.P., S.A. and H.G.A. provided samples. J.F.S. provided samples and contributed to manuscript writing. J.F. made intellectual contributions. R.K. conceived the project, designed the experimental approach, made intellectual contributions and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-15 with Legends, Supplementary Table 1 with patient information, Supplementary Discussion and Supplementary Notes with additional references. (PDF 18859 kb)
Rights and permissions
About this article
Cite this article
Kanasaki, K., Palmsten, K., Sugimoto, H. et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 453, 1117–1121 (2008). https://doi.org/10.1038/nature06951
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06951
This article is cited by
-
MicroRNAs: key regulators of the trophoblast function in pregnancy disorders
Journal of Assisted Reproduction and Genetics (2023)
-
Regulation of mitochondrial dynamics in 2-methoxyestradiol-mediated osteosarcoma cell death
Scientific Reports (2021)
-
Catechol-O-methyltransferase and Pregnancy Outcome: an Appraisal in Rat
Reproductive Sciences (2021)
-
Association Between Endothelial Nitric Oxide Synthase (eNOS) −786 T/C and 27-bp VNTR 4b/a Polymorphisms and Preeclampsia Development
Reproductive Sciences (2021)
-
The Expression and Contribution of SRCs with Preeclampsia Placenta
Reproductive Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.