Abstract
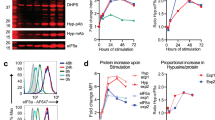
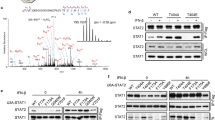
Transcriptional activation of cytokines, such as type-I interferons (interferon (IFN)-α and IFN-β), constitutes the first line of antiviral defence. Here we show that translational control is critical for induction of type-I IFN production. In mouse embryonic fibroblasts lacking the translational repressors 4E-BP1 and 4E-BP2, the threshold for eliciting type-I IFN production is lowered. Consequently, replication of encephalomyocarditis virus, vesicular stomatitis virus, influenza virus and Sindbis virus is markedly suppressed. Furthermore, mice with both 4E- and 4E-BP2 genes (also known as Eif4ebp1 and Eif4ebp2, respectively) knocked out are resistant to vesicular stomatitis virus infection, and this correlates with an enhanced type-I IFN production in plasmacytoid dendritic cells and the expression of IFN-regulated genes in the lungs. The enhanced type-I IFN response in 4E-BP1-/- 4E-BP2-/- double knockout mouse embryonic fibroblasts is caused by upregulation of interferon regulatory factor 7 (Irf7) messenger RNA translation. These findings highlight the role of 4E-BPs as negative regulators of type-I IFN production, via translational repression of Irf7 mRNA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Garcia-Sastre, A. & Biron, C. A. Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312, 879–882 (2006)
Katze, M. G., He, Y. & Gale, M. Viruses and interferon: a fight for supremacy. Nature Rev. Immunol. 2, 675–687 (2002)
Kawai, T. & Akira, S. Innate immune recognition of viral infection. Nature Immunol. 7, 131–137 (2006)
Meylan, E., Tschopp, J. & Karin, M. Intracellular pattern recognition receptors in the host response. Nature 442, 39–44 (2006)
Mathews, M. B., Sonenberg, N. & Hershey, J. W. B. Origins and principles of translational control. In Translational Control in Biology and Medicine (eds Mathews, M. B., Sonenberg, N. & Hershey, J. W. B.) 1–40 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 2007)
Shatkin, A. J. mRNA cap binding proteins: essential factors for initiating translation. Cell 40, 223–224 (1985)
Gingras, A. C., Raught, B. & Sonenberg, N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68, 913–963 (1999)
Sonenberg, N., Morgan, M. A., Merrick, W. C. & Shatkin, A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5′-terminal cap in mRNA. Proc. Natl Acad. Sci. USA 75, 4843–4847 (1978)
Rozen, F. et al. Bidirectional RNA helicase activity of eucaryotic translation initiation factors 4A and 4F. Mol. Cell. Biol. 10, 1134–1144 (1990)
Pestova, T. V., Lorsch, J. R. & Hellen, C. U. T. In Translational Control in Biology and Medicine (eds Mathews, M. B., Sonenberg, N. & Hershey, J. W. B.) 87–128 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 2007)
Pause, A. et al. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 371, 762–767 (1994)
Poulin, F., Gingras, A. C., Olsen, H., Chevalier, S. & Sonenberg, N. 4E–BP3, a new member of the eukaryotic initiation factor 4E-binding protein family. J. Biol. Chem. 273, 14002–14007 (1998)
Hay, N. & Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 (2004)
Mohr, I. J., Pe'ery, T. & Mathews, M. B. Protein synthesis and translational control during viral infection. In Translational Control in Biology and Medicine (eds Mathews, M. B., Sonenberg, N. & Hershey, J. W. B.) 545–600 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 2007)
Le Bacquer, O. et al. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E–BP1 and 4E–BP2. J. Clin. Invest. 117, 387–396 (2007)
Poulin, F., Brueschke, A. & Sonenberg, N. Gene fusion and overlapping reading frames in the mammalian genes for 4E–BP3 and MASK. J. Biol. Chem. 278, 52290–52297 (2003)
Belkowski, L. S. & Sen, G. C. Inhibition of vesicular stomatitis viral mRNA synthesis by interferons. J. Virol. 61, 653–660 (1987)
Colonna, M., Trinchieri, G. & Liu, Y. J. Plasmacytoid dendritic cells in immunity. Nature Immunol. 5, 1219–1226 (2004)
Honda, K. et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 (2005)
Koromilas, A. E., Lazaris-Karatzas, A. & Sonenberg, N. mRNAs containing extensive secondary structure in their 5′ non-coding region translate efficiently in cells overexpressing initiation factor eIF-4E. EMBO J. 11, 4153–4158 (1992)
Honda, K., Takaoka, A. & Taniguchi, T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25, 349–360 (2006)
Stetson, D. B. & Medzhitov, R. Type I interferons in host defense. Immunity 25, 373–381 (2006)
Durbin, J. E., Hackenmiller, R., Simon, M. C. & Levy, D. E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84, 443–450 (1996)
Meraz, M. A. et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84, 431–442 (1996)
Park, C., Li, S., Cha, E. & Schindler, C. Immune response in Stat2 knockout mice. Immunity 13, 795–804 (2000)
Zhou, A. et al. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 16, 6355–6363 (1997)
Kato, H. et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23, 19–28 (2005)
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006)
Kaur, S. et al. Regulatory effects of mammalian target of rapamycin-activated pathways in type I and II interferon signaling. J. Biol. Chem. 282, 1757–1768 (2007)
Sarkar, S. N. et al. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nature Struct. Mol. Biol. 11, 1060–1067 (2004)
Vanhaesebroeck, B., Ali, K., Bilancio, A., Geering, B. & Foukas, L. C. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem. Sci. 30, 194–204 (2005)
Sen, G. C. & Sarkar, S. N. Transcriptional signaling by double-stranded RNA: role of TLR3. Cytokine Growth Factor Rev. 16, 1–14 (2005)
Hiscott, J. et al. Convergence of the NF-κB and interferon signaling pathways in the regulation of antiviral defense and apoptosis. Ann. NY Acad. Sci. 1010, 237–248 (2003)
Honda, K. & Taniguchi, T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nature Rev. Immunol. 6, 644–658 (2006)
Todaro, G. J. & Green, H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17, 299–313 (1963)
Costa-Mattioli, M., Svitkin, Y. & Sonenberg, N. La autoantigen is necessary for optimal function of the poliovirus and hepatitis C virus internal ribosome entry site in vivo and in vitro. Mol. Cell. Biol. 24, 6861–6870 (2004)
Berlanga, J. J. et al. Antiviral effect of the mammalian translation initiation factor 2α kinase GCN2 against RNA viruses. EMBO J. 25, 1730–1740 (2006)
Stojdl, D. F. et al. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J. Virol. 74, 9580–9585 (2000)
Costa-Mattioli, M. et al. eIF2α phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 129, 195–206 (2007)
Stojdl, D. F. et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nature Med. 6, 821–825 (2000)
Irizarry, R. A. et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31, e15 (2003)
Dai, M. et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 33, e175 (2005)
Sandberg, R. & Larsson, O. Improved precision and accuracy for microarrays using updated probe set definitions. BMC Bioinformatics 8, 48 (2007)
Gene Ontology Consortium The Gene Ontology (GO) project in 2006. Nucleic Acids Res. 34, D322–D326 (2006)
Acknowledgements
We thank M. Karin, M. Gale, R. Lin, W. Sossin, L. W. Ler and A. Rosenfeld for comments on the paper, and N. Taheri, A. Sylvestre and C. Lister for assistance. RIG-I and MDA5 antibodies were provided by H. Kato. This work was supported by a grant from the National Cancer Institute of Canada to N.S. and J.C.B. N.S. is a Howard Hughes Medical Institute (HHMI) International scholar. R.C. is supported by a Cole Foundation post-doctoral fellowship and R.J.O.D. is supported by a Terry Fox Foundation studentship.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
The file contains Supplementary Figures S1-S10 with Legends. (PDF 1569 kb)
Rights and permissions
About this article
Cite this article
Colina, R., Costa-Mattioli, M., Dowling, R. et al. Translational control of the innate immune response through IRF-7. Nature 452, 323–328 (2008). https://doi.org/10.1038/nature06730
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06730
This article is cited by
-
Metformin-induced suppression of IFN-α via mTORC1 signalling following seasonal vaccination is associated with impaired antibody responses in type 2 diabetes
Scientific Reports (2020)
-
Ribosome profiling reveals a functional role for autophagy in mRNA translational control
Communications Biology (2020)
-
Transcriptomic analysis of immune response to bacterial lipopolysaccharide in zebra finch (Taeniopygia guttata)
BMC Genomics (2019)
-
MEK inhibition drives anti-viral defence in RV but not RSV challenged human airway epithelial cells through AKT/p70S6K/4E-BP1 signalling
Cell Communication and Signaling (2019)
-
Homoharringtonine induced immune alteration for an Efficient Anti-tumor Response in Mouse Models of Non-small Cell Lung Adenocarcinoma Expressing Kras Mutation
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.