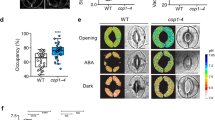
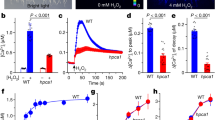
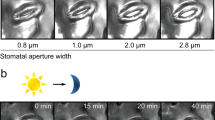
Abstract
The continuing rise in atmospheric [CO2] is predicted to have diverse and dramatic effects on the productivity of agriculture, plant ecosystems and gas exchange1,2,3. Stomatal pores in the epidermis provide gates for the exchange of CO2 and water between plants and the atmosphere, processes vital to plant life4,5,6. Increased [CO2] has been shown to enhance anion channel activity7 proposed to mediate efflux of osmoregulatory anions (Cl– and malate2–) from guard cells during stomatal closure8,9. However, the genes encoding anion efflux channels in plant plasma membranes remain unknown. Here we report the isolation of an Arabidopsis gene, SLAC1 (SLOW ANION CHANNEL-ASSOCIATED 1, At1g12480), which mediates CO2 sensitivity in regulation of plant gas exchange. The SLAC1 protein is a distant homologue of bacterial and fungal C4-dicarboxylate transporters, and is localized specifically to the plasma membrane of guard cells. It belongs to a protein family that in Arabidopsis consists of four structurally related members that are common in their plasma membrane localization, but show distinct tissue-specific expression patterns. The loss-of-function mutation in SLAC1 was accompanied by an over-accumulation of the osmoregulatory anions in guard cell protoplasts. Guard-cell-specific expression of SLAC1 or its family members resulted in restoration of the wild-type stomatal responses, including CO2 sensitivity, and also in the dissipation of the over-accumulated anions. These results suggest that SLAC1-family proteins have an evolutionarily conserved function that is required for the maintenance of organic/inorganic anion homeostasis on the cellular level.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hetherington, A. M. & Woodward, F. I. The role of stomata in sensing and driving environmental change. Nature 424, 901–908 (2003)
Sellers, P. J. et al. Modeling the exchanges of energy, water, and carbon between continents and the atmosphere. Science 275, 502–509 (1997)
Shaw, M. R. et al. Grassland responses to global environmental changes suppressed by elevated CO2 . Science 298, 1987–1990 (2002)
MacRobbie, E. A. C. Signal transduction and ion channels in guard cells. Phil. Trans. R. Soc. Lond. B 353, 1475–1488 (1998)
Hetherington, A. M. Guard cell signaling. Cell 107, 711–714 (2001)
Schroeder, J. I. et al. Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658 (2001)
Raschke, K., Shabahang, M. & Wolf, R. The slow and the quick anion conductance in whole guard cells: their voltage-dependent alternation, and the modulation of their activities by abscisic acid and CO2 . Planta 217, 639–650 (2003)
Schroeder, J. I. & Hagiwara, S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338, 427–430 (1989)
Willmer, C. M. & Fricker, M. D. Stomata 2nd edn (Chapman and Hall, London, 1996)
Hashimoto, M. et al. Arabidopsis HT1 kinase controls stomatal movements in response to CO2 . Nature Cell Biol. 8, 391–397 (2006)
Merlot, S. et al. Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 30, 601–609 (2002)
Xie, X. D. et al. The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Curr. Biol. 16, 882–887 (2006)
Negi, J., Hashimoto, M. & Iba, K. Characterization of CO2–insensitive Arabidopsis mutant cdi3. Plant Cell Physiol. 46, s176 (2005)
Vahisalu, T. et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature (this issue) 10.1038/nature06608
Uemura, T., Yoshimura, S., Takeyasu, K. & Sato, M. Vacuolar membrane dynamics revealed by GFP–AtVam3 fusion protein. Genes Cells 7, 743–753 (2002)
Saier, M. H. et al. Phylogenetic characterization of novel transport protein families revealed by genome analyses. Biochim. Biophys. Acta 1422, 1–56 (1999)
Grobler, J., Bauer, F., Subden, R. E. & van Vuuren, H. J. The mae1 gene of Schizosaccharomyces pombe encodes a permease for malate and other C4 dicarboxylic acids. Yeast 11, 1485–1491 (1995)
Pfam database. 〈http://pfam.sanger.ac.uk/family?acc=PF03595〉 (2007)
Vavasseur, A. & Raghavendra, A. Guard cell metabolism and CO2 sensing. New Phytol. 165, 665–682 (2005)
Outlaw, D. et al. Requirements for activation of the signal-transduction network that leads to regulatory phosphorylation of leaf guard-cell phosphoenolpyruvate carboxylase during fusicoccin-stimulated stomatal opening. Arch. Biochem. Biophys. 407, 63–71 (2002)
TMHMM Server v.2.0 〈http://www.cbs.dtu.dk/services/TMHMM/〉 (2007)
De Angeli, A. et al. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442, 939–942 (2006)
Xu, J. et al. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125, 1347–1360 (2006)
Ryan, P. R., Delhaize, E. & Jones, D. L. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 527–560 (2001)
Köhler, B. & Raschke, K. The delivery of salts to the xylem. Three types of anion conductance in the plasmalemma of the xylem parenchyma of roots of barley. Plant Physiol. 122, 243–254 (2000)
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998)
Mustilli, A. C., Merlot, S., Vavasseur, A., Fenzi, F. & Giraudat, J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14, 3089–3099 (2002)
Inada, H. & Iba, K. in Photosynthesis: Mechanisms and Effects (ed. Garab, G.) 4 2999–3002 (Dordrecht, London, 1998)
Chiu, W. et al. Engineered GFP as a vital reporter in plants. Curr. Biol. 6, 325–330 (1996)
Dong, J., Kim, S. T. & Lord, E. M. Plantacyanin plays a role in reproduction in Arabidopsis. Plant Physiol. 138, 778–789 (2005)
Hirata, N., Yonekura, D., Yanagisawa, S. & Iba, K. Possible involvement of the 5′-flanking region and the 5′UTR of plastid accD gene in NEP-dependent transcription. Plant Cell Physiol. 45, 176–186 (2004)
Pandey, S., Wang, X. Q., Coursol, S. A. & Assmann, S. M. Preparation and applications of Arabidopsis thaliana guard cell protoplasts. New Phytol. 153, 517–526 (2002)
Ueno, K. et al. Biochemical characterization of plasma membrane H+-ATPase activation in guard cell protoplasts of Arabidopsis thaliana in response to blue light. Plant Cell Physiol. 46, 955–963 (2005)
Gotow, K. et al. Light activation of NADP-malate dehydrogenase in guard cell protoplasts from Vicia faba. Plant Physiol. 79, 829–832 (1985)
Acknowledgements
We thank M. H. Sato for providing the tonoplast control plants, I. C. Mori for technical advice, and N. Kawahara for technical assistance. This research was supported by a Core Research for Evolution and Technology (CREST)-type JST grant and a JSPS grant, and by the Ministry of Agriculture, Forestry and Fisheries of Japan (Rice Genome Project IP-5005) grants (K.I.). J.N. is the recipient of scholarships from JSPS and Nara Institute of Science and Technology (NAIST). M.H. is the JST post-doctoral fellow.
Author information
Authors and Affiliations
Corresponding author
Additional information
Arabidopsis Genome Initiative (AGI) identifiers for SLAC1-family members are: SLAC1, At1g12480; SLAH1, At1g62280; SLAH2, At4g27970; and SLAH3, At5g24030.
Supplementary information
Supplementary Figures
The file contains Supplementary Figures S1-S5 with Legends. (PDF 577 kb)
Rights and permissions
About this article
Cite this article
Negi, J., Matsuda, O., Nagasawa, T. et al. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452, 483–486 (2008). https://doi.org/10.1038/nature06720
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06720
This article is cited by
-
Genome-wide identification and expression analysis of the NRT genes in Ginkgo biloba under nitrate treatment reveal the potential roles during calluses browning
BMC Genomics (2023)
-
Balancing nitrate acquisition strategies in symbiotic legumes
Planta (2023)
-
Plant hormone regulation of abiotic stress responses
Nature Reviews Molecular Cell Biology (2022)
-
New functions of CIPK gene family are continue to emerging
Molecular Biology Reports (2022)
-
Salicylic Acid Increases Photosynthesis of Drought Grown Mustard Plants Effectively with Sufficient-N via Regulation of Ethylene, Abscisic Acid, and Nitrogen-Use Efficiency
Journal of Plant Growth Regulation (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.