Abstract
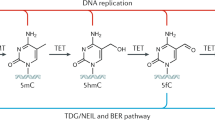
Processes that regulate gene transcription are directly under the influence of the genome organization. The epigenome contains additional information that is not brought by DNA sequence, and generates spatial and functional constraints that complement genetic instructions. DNA methylation on CpGs constitutes an epigenetic mark generally correlated with transcriptionally silent condensed chromatin. Replication of methylation patterns by DNA methyltransferases maintains genome stability through cell division. Here we present evidence of an unanticipated dynamic role for DNA methylation in gene regulation in human cells. Periodic, strand-specific methylation/demethylation occurs during transcriptional cycling of the pS2/TFF1 gene promoter on activation by oestrogens. DNA methyltransferases exhibit dual actions during these cycles, being involved in CpG methylation and active demethylation of 5mCpGs through deamination. Inhibition of this process precludes demethylation of the pS2 gene promoter and its subsequent transcriptional activation. Cyclical changes in the methylation status of promoter CpGs may thus represent a critical event in transcriptional achievement.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Spector, D. L. The dynamics of chromosome organization and gene regulation. Annu. Rev. Biochem. 72, 573–608 (2003)
Casolari, J. M., Brown, C. R., Drubin, D. A., Rando, O. J. & Silver, P. A. Developmentally induced changes in transcriptional program alter spatial organization across chromosomes. Genes Dev. 19, 1188–1198 (2005)
Khorasanizadeh, S. The nucleosome: from genomic organization to genomic regulation. Cell 116, 259–272 (2004)
Dillon, N. Gene regulation and large-scale chromatin organization in the nucleus. Chromosome Res. 14, 117–126 (2006)
Klose, R. J. & Bird, A. P. Genomic DNA methylation: the mark and its mediators. Trends Biochem. Sci. 31, 89–97 (2006)
Oswald, J. et al. Active demethylation of the paternal genome in the mouse zygote. Curr. Biol. 10, 475–478 (2000)
Murayama, A. et al. A specific CpG site demethylation in the human interleukin 2 gene promoter is an epigenetic memory. EMBO J. 25, 1081–1092 (2006)
Rougier, N. et al. Chromosome methylation patterns during mammalian preimplantation development. Genes Dev. 12, 2108–2113 (1998)
Matsuo, K. et al. An embryonic demethylation mechanism involving binding of transcription factors to replicating DNA. EMBO J. 17, 1446–1453 (1998)
Morales-Ruiz, T. et al. Demeter and Repressor of Silencing 1 encode 5-methylcytosine DNA glycosylases. Proc. Natl Acad. Sci. USA 103, 6853–6858 (2006)
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007)
Becker, P. B. & Horz, W. ATP-dependent nucleosome remodeling. Annu. Rev. Biochem. 71, 247–273 (2002)
Shang, Y., Hu, X., DiRenzo, J., Lazar, M. A. & Brown, M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103, 843–852 (2000)
Metivier, R. et al. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115, 751–763 (2003)
Robinson-Rechavi, M., Escriva Garcia, H. & Laudet, V. The nuclear receptor superfamily. J. Cell Sci. 116, 585–586 (2003)
Brzozowski, A. M. et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389, 753–758 (1997)
Zhang, Y. et al. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 13, 1924–1935 (1999)
Harikrishnan, K. N. et al. Brahma links the SWI/SNF chromatin-remodeling complex with MeCP2-dependent transcriptional silencing. Nature Genet. 37, 254–264 (2005)
Christman, J. K. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 21, 5483–5495 (2002)
Brueckner, B. et al. Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases. Cancer Res. 65, 6305–6311 (2005)
Sharma, D., Saxena, N. K., Davidson, N. E. & Vertino, P. M. Restoration of tamoxifen sensitivity in estrogen receptor-negative breast cancer cells: tamoxifen-bound reactivated ER recruits distinctive corepressor complexes. Cancer Res. 66, 6370–6378 (2006)
Zingg, J. M., Shen, J. C., Yang, A. S., Rapoport, H. & Jones, P. A. Methylation inhibitors can increase the rate of cytosine deamination by (cytosine-5)-DNA methyltransferase. Nucleic Acids Res. 24, 3267–3275 (1996)
Sharath, A. N., Weinhold, E. & Bhagwat, A. S. Reviving a dead enzyme: cytosine deaminations promoted by an inactive DNA methyltransferase and an S-adenosylmethionine analogue. Biochemistry 39, 14611–14616 (2000)
Waters, T. R., Gallinari, P., Jiricny, J. & Swann, P. F. Human thymine DNA glycosylase binds to apurinic sites in DNA but is displaced by human apurinic endonuclease 1. J. Biol. Chem. 274, 67–74 (1999)
Li, Y. Q., Zhou, P. Z., Zheng, X. D., Walsh, C. P. & Xu, G. L. Association of Dnmt3a and thymine DNA glycosylase links DNA methylation with base-excision repair. Nucleic Acids Res. 35, 390–400 (2007)
Gallais, R. et al. Deoxyribonucleic acid methyl transferases 3a and 3b associate with the nuclear orphan receptor COUP-TFI during gene activation. Mol. Endocrinol. 21, 2085–2098 (2007)
Endoh, H. et al. Purification and identification of p68 RNA helicase acting as a transcriptional coactivator specific for the activation function 1 of human estrogen receptor alpha. Mol. Cell. Biol. 19, 5363–5372 (1999)
Jost, J. P. et al. A chicken embryo protein related to the mammalian DEAD box protein p68 is tightly associated with the highly purified protein-RNA complex of 5-MeC-DNA glycosylase. Nucleic Acids Res. 27, 3245–3252 (1999)
Suetake, I., Miyazaki, J., Murakami, C., Takeshima, H. & Tajima, S. Distinct enzymatic properties of recombinant mouse DNA methyltransferases Dnmt3a and Dnmt3b. J. Biochem. 133, 737–744 (2003)
Fritah, A., Redeuilh, G. & Sabbah, M. Molecular cloning and characterization of the human WISP-2/CCN5 gene promoter reveal its upregulation by oestrogens. J. Endocrinol. 191, 613–624 (2006)
Fatemi, M., Hermann, A., Gowher, H. & Jeltsch, A. Dnmt3a and Dnmt1 functionally cooperate during de novo methylation of DNA. Eur. J. Biochem. 269, 4981–4984 (2002)
Chen, D. et al. T:G mismatch-specific thymine-DNA glycosylase potentiates transcription of estrogen-regulated genes through direct interaction with estrogen receptor alpha. J. Biol. Chem. 278, 38586–38592 (2003)
Jost, J. P., Thiry, S. & Siegmann, M. Estradiol receptor potentiates, in vitro, the activity of 5-methylcytosine DNA glycosylase. FEBS Lett. 527, 63–66 (2002)
Zhu, B. et al. 5-methylcytosine-DNA glycosylase activity is present in a cloned G/T mismatch DNA glycosylase associated with the chicken embryo DNA demethylation complex. Proc. Natl Acad. Sci. USA 97, 5135–5139 (2000)
Fuks, F. DNA methylation and histone modifications: teaming up to silence genes. Curr. Opin. Genet. Dev. 15, 490–495 (2005)
Oka, M., Rodic, N., Graddy, J., Chang, L. J. & Terada, N. CpG sites preferentially methylated by Dnmt3a in vivo. J. Biol. Chem. 281, 9901–9908 (2006)
Li, F. et al. Chimeric DNA methyltransferases target DNA methylation to specific DNA sequences and repress expression of target genes. Nucleic Acids Res. 35, 100–112 (2007)
Lucarelli, M., Fuso, A., Strom, R. & Scarpa, S. The dynamics of myogenin site-specific demethylation is strongly correlated with its expression and with muscle differentiation. J. Biol. Chem. 276, 7500–7506 (2001)
Kress, C., Thomassin, H. & Grange, T. Active cytosine demethylation triggered by a nuclear receptor involves DNA strand breaks. Proc. Natl Acad. Sci. USA 103, 11112–11117 (2006)
Barreto, G. et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 445, 671–675 (2007)
Leteurtre, F. et al. Effects of DNA methylation on topoisomerase I and II cleavage activities. J. Biol. Chem. 269, 7893–7900 (1994)
Ju, B. G. et al. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science 312, 1798–1802 (2006)
Lamm, G. M., Nicol, S. M., Fuller-Pace, F. V. & Lamond, A. I. p72: a human nuclear DEAD box protein highly related to p68. Nucleic Acids Res. 24, 3739–3947 (1996)
Gowher, H., Liebert, K., Hermann, A., Xu, G. & Jeltsch, A. Mechanism of stimulation of catalytic activity of Dnmt3A and Dnmt3B DNA-(cytosine-C5)-methyltransferases by Dnmt3L. J. Biol. Chem. 280, 13341–13348 (2005)
Gowher, H. et al. Mutational analysis of the catalytic domain of the murine Dnmt3a DNA-(cytosine C5)-methyltransferase. J. Mol. Biol. 357, 928–941 (2006)
Metivier, R. et al. Transcriptional complexes engaged by apo-oestrogen receptor-alpha isoforms have divergent outcomes. EMBO J. 23, 3653–3666 (2004)
Nelson, J. E. & Krawetz, S. A. Purification of cloned and genomic DNA by guanidine thiocyanate/isobutyl alcohol fractionation. Anal. Biochem. 207, 197–201 (1992)
Acknowledgements
We thank B. Brueckner for the gift of the RG108 compound, P. Chambon for the pSG5-TDGwt plasmid, F. Fuller-Pace for the anti-p68 RNA helicase antibody, and V. Legagneux and U. K. Laemmli for the anti-CAPD2 and anti-CAPH antibodies, respectively. This work was supported by funds from the Ministère de l’Education Nationale de l’Enseignement Supérieur et de la Recherche (MENESR), the Centre National de la Recherche Scientifique (CNRS), the University of Rennes I, the Association pour la Recherche contre le Cancer (ARC), the Ligue contre le Cancer, and by funding from EMBO and EMBL. R.G. was supported by a grant from the MENESR and a fellowship from the Ligue Nationale Contre le Cancer. We also thank C. Ralliere for DNA sequencing, C. Martin, F. Percevault and C. Tascon for their technical assistance, and B.Stride and S. Kangaspeska for their comments during the writing of the manuscript.
Authors Contributions ChIP, sequential-ChIP experiments, bisulphite and run-on assays were performed by R.M. Methylation/deamination assays were set up by R.G. and performed by R.G. and R.M. C.T. ran all RT-PCR experiments on siRNA-treated cells, and set up the analysis on the Wisp-2 gene. Co-immunoprecipitations and proteomic controls were performed by C.L.P., R.G. and R.M. Synthesis and purification of TDG protein were performed by P.B. and F.D., as the preparation of the anti-TDG antibody. R.Z.J. purified all the Dnmt3 catalytic domains. R.P.C. and D.I. were involved, under the supervision of V.B., in the mass sequencing of the clones generated during the bisulphite-mediated analysis of CpG methylation and through the in vitro deamination assays. G.R. introduced R.M. to FACS analysis and bisulphite-modification of DNA. R.M., G.R., A.J., F.G. and G.S. were responsible for the overall project management, strategy and data interpretation. R.M., G.R. and G.S. prepared the manuscript. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
The file contains Supplementary Discussion with additional references, Supplementary Figures S1 to S10 with Legends and Supplementary Tables 1 to 4. Supplementary Figures and Supplementary Tables present additional controls and data that support both the main text and supplementary discussion. Supplementary Figure S10 depicts a scheme summarizing the proposed mechanism. Supplementary Figures 1a, 3d, 7c, e and 8a-d were replaced on 21 Jan 2010. (PDF 15174 kb)
Rights and permissions
About this article
Cite this article
Métivier, R., Gallais, R., Tiffoche, C. et al. Cyclical DNA methylation of a transcriptionally active promoter. Nature 452, 45–50 (2008). https://doi.org/10.1038/nature06544
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06544
This article is cited by
-
DNA methylation haplotype block signatures responding to Staphylococcus aureus subclinical mastitis and association with production and health traits
BMC Biology (2024)
-
DNA methylation signatures on vascular differentiation genes are aberrant in vessels of human cerebral arteriovenous malformation nidus
Clinical Epigenetics (2022)
-
Fluctuating methylation clocks for cell lineage tracing at high temporal resolution in human tissues
Nature Biotechnology (2022)
-
Enriched environment causes epigenetic changes in hippocampus and improves long-term cognitive function in sepsis
Scientific Reports (2022)
-
Epigenetics: a new warrior against cardiovascular calcification, a forerunner in modern lifestyle diseases
Environmental Science and Pollution Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.